Product Compliance
filing/registration/consulting/training

November 1, 2023,The US Food and Drug Administration (FDA) Issued Draft Guidance on Registration and Listing of Cosmetic Product Facilities and Products official vision, which provides more detail on the requirements for facility registration and product listing under the Modernization of Cosmetic Regulations Act of 2022 (MoCRA). Cosmetics exported to the United States require facility registration and product listing.
*Facility Registration: Facility registration, it can be simply understood as factory registration,The following will replace facility registration with factory registration,Hereby declare that.
Compliance process

Cosmetic Compliance Process
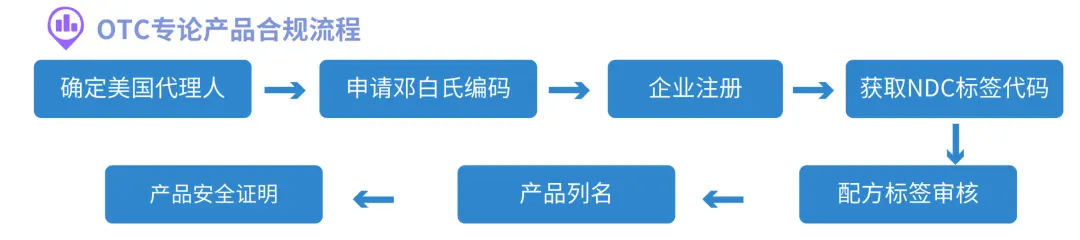
OTC Product Compliance Process
Required information for factory registration
Factory (ordinary cosmetics): Enterprises (including overseas enterprises) involved in the production and processing of cosmetics sold in the United States;
Factory (OTC): All enterprises engaged in the production, repackaging, relabeling or recycling of drugs for commercial sales must register with the FDA.
1. Factory business license;
2. US FDA Cosmetic Good Manufacturing Practice (GMP Certificate) / Current Good Manufacturing Practice (cGMP Certificate) (OTC);
3. For any factory outside the United States, the contact information (name and telephone number) and electronic contact information (email, if any) of the factory's US agent;
4. The brand name, product name and responsible person information of the cosmetics produced or processed by the factory.
Information required for product listing
Responsible person (ordinary cosmetics): the cosmetics manufacturer, packager or distributor that appears on the cosmetics label;
Responsible person (OTC): manufacturer, repacker, relabeler or private label distributor (PLD).
1. Business license;
2. Name and contact information of the responsible person: must be consistent with that shown on the label;
3. Product formula table;
* In addition, there are some non-mandatory information to be submitted, such as labels, product website links, whether the product is for professional use only, etc.
National drug code(NDC) NDCRequired information for label codes(OTC)
Responsible person OTC Manufacturer Repackaging Re labeling or self branded distributors Private Label Distributor, PLD .
◆Name of registered enterprise or self owned brand distributor,Actual address,Deng Bai's code
◆The name of the contact person,Mailing address,Phone number:,E-mail
◆Name of US agent,Phone number:,E-mail,Deng Bai's code.
Professional services that Zongpu can provide
◆Applying for Deng Bai's code DUNSnumber
◆Ordinary cosmetics andFDA OTCSpecial Discussion on Product Enterprise Registration
◆Product formula and label review
◆Ordinary cosmetics andOTCMonograph product listing
◆Annual update services for enterprise registration and product listing
◆Testing and security assessment services required for product safety certification
◆Undertake local agency in the United States As needed
◆Cosmetic classification and definition service
◆US Cosmetic Regulatory Compliance Training
◆Customized services for other customers
Apply for Dun & Bradstreet number (DUNS number)
Registration of general cosmetics and FDA OTC monograph products
Product formula and label review
Listing of general cosmetics and OTC monograph products
Annual update service for company registration and product listing
Testing and safety assessment service required for product safety certification
Acting as a local agent in the United States (as needed)
Cosmetic classification and definition service
US cosmetics regulatory compliance training
Other customized services
The advantages of ZOOP
Since its establishment in 2010, ZOOP focuses on product compliance-related services. The company's cosmetic division audit teachers are all from medium- to large-sized cosmetic manufacturers and have relevant professional backgrounds in biology, medicine, cosmetics and chemistry.
Our advantages are:
1. Professional and mature team, periodic service guarantee;
2. Reasonable prices, high-quality services throughout the process, and peace of mind;
3. With outlets in Shenzhen, Guangzhou, Shanghai and Beijing, communication is convenient;
4. One-stop service for makeup , disinfection and medical devices , efficient and cost-effective;
5. Can provide services in full English to meet the needs of domestic and foreign customers ;
Responsible for customers and quality service are the consistent business philosophy of ZOOP. We hope to serve more quality-focused customers and grow with you!