Product Compliance
filing/registration/consulting/training

On June 29, 2020, The State Council of the People's Republic of China issued the Regulations on the Supervision and Administration of Cosmetics (effective from January 1, 2021), which clearly declares that cosmetics with new effects are special-use cosmetics. The NMPA under The State Council shall formulate and publish rules and catalogues for the classification of cosmetics according to such factors as cosmetic's efficacy, used part, dosage forms and users.
Cosmetics declaring new effects shall be registered with the NMPA under The State Council before being produced and marketed or imported for the first time, and registration certificates shall be obtained before being produced and imported.
The efficacy claims of cosmetics should have sufficient scientific basis, such as research data and evaluation conclusions obtained through human efficacy evaluation tests, laboratory tests, consumer use tests, and relevant scientific literature materials.
1. Cosmetics other than the following claims: hair dying, perming, freckle-removal, whitening, sunscreen, anti-hair loss, acne removal, nourishing, repairing, cleansing, makeup remover, moisturizing, beauty modification, fragrance, deodorant, anti-wrinkle, firming, soothing, oil control , Exfoliation, body refreshing, hair care, anti-breaking, anti-dandruff, hair-color care, hair removal, shaving auxiliary ;
2. Cosmetics that act on areas other than the following part: hair, body hair, torso, head, face, eyes, lips, hands, feet, whole body skin, nails;
3. Cosmetics claimed to be suitable for pregnant and lactating women.
Legal Accordance
Regulations on the Supervision and Administration of Cosmetics,Administration for Cosmetics Registration and Filing,Classification Rules and Catalogues of Cosmetics,Evaluation Standards for Cosmetic Efficacy Claims,NMPA Announcement on Issuing and Implementing Regulations for the Registration and Filing of Cosmetics Inspection (No. 72, 2019),Regulations on the Administration of Cosmetics Registration and Filing Documents.
Registration process
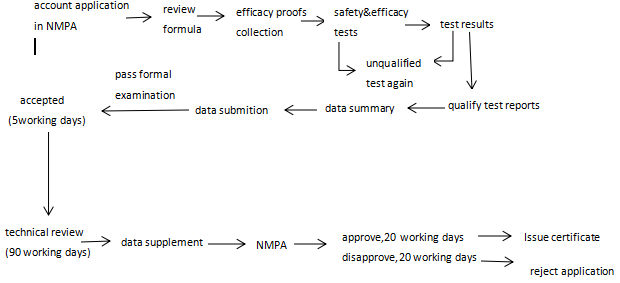
Qualification of Registrant or Filing Applicant
The registrant or the filing applicant shall meet the following basic conditions:
(1) it shall have a quality management system suitable for cosmetics to be registered or filed, and a person in charge of quality safety who has professional knowledge of cosmetics quality safety and has more than 5 years of experience in cosmetics production or quality management;
(2) It shall have management systems such as supplier selection, raw material acceptance, production process and quality control, equipment management, product inspection and sample retention, which are suitable for cosmetics to be registered and filing;
(3) It shall have safety risk assessment, adverse reaction monitoring and evaluation and recall systems corresponding to the cosmetics to be registered and filing.
Where the registrant or filing applicant is an overseas enterprise, an enterprise legal person within the territory of China shall be designated as the domestic responsible person,and the person shall perform the following obligations:
(1) To register cosmetics in the name of the applicant and put the products into the domestic market;
(2) Assist the registrant in the adverse reactions monitoring of cosmetics, product recall, safety monitoring and reporting;
(3) To cooperate with the supervision and inspection work of the regulatory department.
Required Dossiers:
1. Cosmetics Registration and Filing Information Form and relevant materials .
2. Product name information.
3. Product formula((including name and CAS number of raw material ,percentage, purpose of use, safety information).
4. Carried standard for the product.
5. Product label draft.
6. Product test reports(including microbiology and physical and chemical tests, toxicological tests, human safety tests and human efficacy test reports.).
7. Product safety assessment.
when registering,the summary of the basis for the product efficacy claim shall be uploaded to the website designated by the NMPA (starting from 1 January 2022).
If the conditions for alteration are met, alteration for the registration certificate may be applied for.
The registration certificate for domestic special-use cosmetics is valid for 5 years, and the holder shall apply for extension 90 working days to 30 working days before the expiry of the registration certificate.Expiration is automatically invalidated and shall be reapplied.
Professional Services Provided by ZOOP:
(1) Coaching the establishment of quality management system and checking the qualification of the head of quality and safety ;
(2) Counseling to establish an adverse reaction monitoring and evaluation system;
(3) Pre-examination of production capacity ;
(4) Review of formula compliance;
(5) Compilation of product implementation standards;
(6)Review of label compliance;
(7)Product tests on safety and efficacy;
(8)Compilation of product safety assessment report;
(9)Collect product efficacy literature;
(10)Full agent services for registration.
ZOOP Advantages:
Since its establishment in 2010, ZOOP has focused on product compliance related services. The company's technical team consists of personnel with biomedical, cosmetic, chemical and other related professional backgrounds, who are familiar with the review dynamic of cosmetics regulations. Meanwhile, the company's mature process management of product registration and filing makes the service and cycle more guaranteed.