Product Compliance
filing/registration/consulting/training

I. Definition of Cosmetics in the Philippines
"Filing for cosmetics in the Philippines" is a critical step to ensure compliance with FDA regulations. According to the definition by the Philippine Food and Drug Administration (FDA), cosmetics are "any substance or preparation intended to be applied to the external parts of the human body (such as the epidermis, hair system, nails, lips, and external genitalia), or to the teeth and oral mucosa, with the sole or primary purpose of cleaning, perfuming, altering appearance, and/or eliminating body odor, and/or protecting or maintaining these parts in good condition." This definition includes skincare creams, shampoos, perfumes, lipsticks, makeup, mouthwashes, toothpaste, oral sprays, feminine washes, and more.
II. Regulatory Background
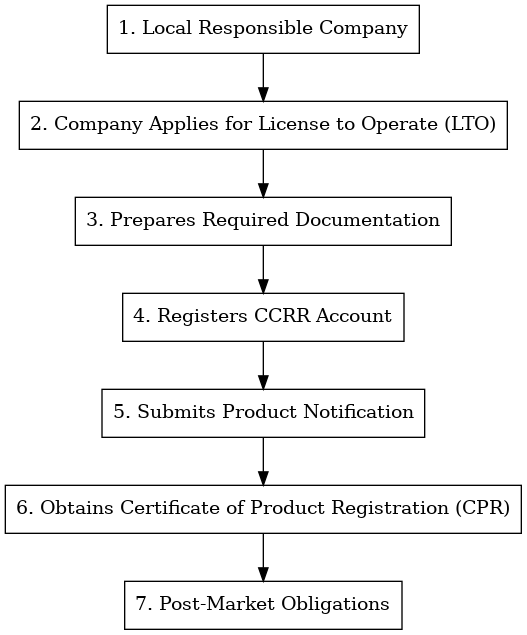
In the Philippines, the Food and Drug Administration (FDA), formerly the Bureau of Food and Drugs, operates under the Department of Health and is responsible for regulating cosmetic products to ensure their safety, purity, and quality in the Philippine market. As a key member of the ASEAN, the Philippines formally incorporated the ASEAN Harmonized Cosmetic Regulatory Scheme (ACRS) and the ASEAN Common Technical Dossier (ACTD) into its national regulations in April 2005, granting the cosmetics industry a transition period until December 31, 2007. Since then, the Philippines has implemented a notification system for cosmetic products, where the notifying entity must submit applications through the FDA E-Portal using a CCRR user account (issued by the FDA).
All cosmetics companies must obtain a License to Operate (LTO) before engaging in the production, importation, distribution, or sale of cosmetics. The LTO is also a prerequisite for filing cosmetic product notifications. Companies must ensure that their notified products comply with the relevant requirements of the ASEAN Cosmetic Directive (ACD) and its annexes and supplements.
III. Market Entry Process
IV. Required Documentation for Notification
Basic Product Information
Brand name
Product name
Variants/packaging sizes/container types
Net content
Formula table (INCI names, ingredient functions, concentrations)
Notifying Entity Information (must match the LTO)
Company name and address
License to Operate number (LTO No.)
Nature of the notifying entity: distributor, manufacturer, or trader
Manufacturer’s name and address
Name and contact number of the company representative
Supporting Documents (potential supplementary materials)
Product label samples
Usage instructions
Mechanism of action
Certificate of origin for ingredients
Material Safety Data Sheet (MSDS for the product or specific ingredients)
Certificate of Analysis (COA for the product or specific ingredients)
V. Notification Timeline
Product notification: 3–4 months
Notification validity: 1, 2, or 3 years (subject to QPIRA qualification restrictions)
VI. Product Information File (PIF)
The PIF includes the following four main sections:
Administrative documents
Quality and safety data of cosmetic ingredients—raw material quality and safety information
Product quality and safety data
Product safety and efficacy data—e.g., safety assessment reports, test reports, efficacy reports, etc.
Other potential supporting documents
VII. Professional Services Provided by ZOOP
Provision of a local agent (notifying entity)
License to Operate (LTO) application
Packaging, labeling, and formula review services
Product notification services (review and submission)
PIF review and compilation services
Product testing, safety assessment (CPSR), MSDS, and other services
Why Choose Us?
14 years of compliance experience, a professional and mature team with guaranteed service timelines
Reasonable pricing and high-quality end-to-end service
Offices in Shenzhen, Guangzhou, Shanghai, and Beijing, serving global clients from China
One-stop services for cosmetics, disinfectants, and medical devices, ensuring efficiency and cost savings
Full English communication to meet the needs of domestic and international clients
Choose ZOOP, choose trust!