Product Compliance
filing/registration/consulting/training

Definition
Toothpaste refers to solid and semi solid preparations used for the purpose of cleaning, beautifying, and protecting the surface and surrounding tissues of human teeth by friction.
Toothpaste testing requirements
According to regulations: Toothpaste needs to refer to the supervision of ordinary cosmetics, and products need to be filed and publicized by the drug regulatory department before they can be marketed. Toothpaste filing requires completing necessary testing and efficacy evaluation, and submitting corresponding testing and evaluation reports. The details are as follows:
1. Basic testing items and requirements
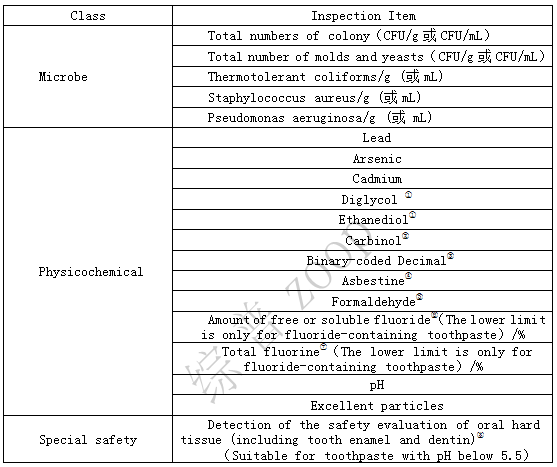
Note:
① Products containing glycerol, propylene glycol, or polyethylene glycol in the formula should be tested for diethylene glycol and ethylene glycol. (In the finished toothpaste, the sum of diethylene glycol and ethylene glycol brought in from other raw materials as magazines should be less than or equal to 0.1%.)
② For products with a total content of ethanol and isopropanol ≥ 10% (w/w), methanol testing is required.
③ Products containing ethoxy structural raw materials in the formula need to be tested for dioxane items.
④ Products containing talc powder in the formula need to be tested for asbestos items.
⑤ Products containing formaldehyde releasing substances in the formula need to be tested for free formaldehyde.
⑥ The formula contains fluoride and requires testing for free or soluble fluoride content items. Fluoride containing toothpaste using sodium monofluorophosphate or sodium monofluorophosphate in combination with sodium fluoride (stannous fluoride, ammonium fluoride) is suitable for the detection of soluble fluoride; Fluoride toothpaste made from sodium fluoride or (stannous fluoride, ammonium fluoride) is suitable for the detection of free fluorine. If more fluoride is used than sodium monofluorophosphate, sodium fluoride, stannous fluoride, and ammonium fluoride, appropriate detection methods should be selected.
⑦ The formula contains fluoride, and the total fluoride content item needs to be tested.
⑧ For products with a pH lower than 5.5, the registrant should provide an inspection report published by the State Drug Administration on the safety evaluation of oral hard tissue (including enamel and dentin) by an inspection and testing agency.
2. Allowable claims and efficacy evaluation requirements for toothpaste
Code1 | Class | List of allowable terms for toothpaste efficacy claims | Efficacy evaluation requirements | Declarable population |
01 | The class of foundation cleaning | Clean teeth/oral cavity, maintain oral hygiene, maintain oral health, reduce soft dirt, freshen breath, and refreshing taste. | No efficacy evaluation and summary | Enfant/Adults |
02 | The class of caries prevention | Prevent dental caries, strengthen teeth, prevent caries, prevent cavities, inhibit tooth demineralization, and promote tooth remineralization. | 1. Fluoride anti-caries toothpaste is exempted from the clinical evaluation of anti-caries efficacy; 2. Non-fluoride anti-caries toothpaste and other efficacy should be evaluated in accordance with "toothpaste supervision and management methods", national standards, industry standards, technical specifications of the requirements of clinical evaluation methods. | Enfant/Adults |
03 | The class of inhibition of dental plaque | Inhibit dental plaque and reduce plaque formation. | Adults | |
04 | The class of anti-dentin-sensitive | Anti dentin hypersensitivity, alleviate the stimulation of hot and cold sour sweets on teeth, alleviate the stimulation of hot and cold sour sweets, alleviate tooth sensitivity, alleviate sensitivity, resist allergy, resist allergy, relieve allergy, repair sensitive teeth, and close dentin tubules. | Adults | |
05 | The class of reducing gum problems | Clear fire, dispel fire, improve gum health, and protect gums. | Adults | |
06 | Whitening class | Whiten teeth, whiten teeth, whiten teeth through oxidation reactions, reduce exogenous coloration, mechanically reduce exogenous coloration, chemically reduce exogenous coloration, and achieve whitening/whitening effects through optical effects. | The methods specified in relevant domestic and foreign regulations, technical standards, technical specifications, authoritative organizations or technical institutions, industry association guidelines, and professional academic journals can be selected for public publication.If a laboratory developed method is used, the method should be validated in accordance with the CMA/CNAS method validation process, and the complete text of the method should be prepared in the evaluation report. | Adults |
07 | The class of anti-calculus | Reduce dental calculus, reduce dental calculus, decompose dental calculus, reduce dental calculus formation, prevent dental calculus, and inhibit dental calculus formation. | Adults | |
08 | The class of reducing bad breath | Reduce bad breath, alleviate bad odors, remove volatile sulfide compounds, and inhibit odorogenic bacteria that produce breath. | Adults | |
09 | Other efficacy | Moisturize the mouth and improve dry mouth condition. | Adults |
Professional services that Zoop can provide
In order to meet the needs of manufacturers for product testing, relying on rich professional knowledge and laboratory resources, Comprehensive Consulting can provide manufacturers with services ranging from basic testing to clinical evaluation:
1. Filing and testing services;
2. General entrusted testing services;
3. Special efficacy evaluation and testing program development and testing services;
4. Clinical evaluation of overall agency efficacy;
5. Other customer specified project testing services.
Service advantages of Zoop
1. Abundant laboratory resources to meet different testing requirements for prices and cycles;
2. Experienced, familiar with drug regulatory review requirements and various efficacy testing methods;
3. Full process service, more attentive.
4. A completely independent third-party organization with standardized internal management throughout the entire process, which does not involve the production and operation of cosmetics and is safe and secure;