Product Compliance
filing/registration/consulting/training

There are some differences in cosmetic labeling requirements between the United States and the European Union,Mainly reflected in the details of the labels.in the U.S.,The label needs to include a main display panel PDP ,The side that is directly displayed to consumers.PDPBrand name must be indicated on the label Product Name and Net Content,The net content should be in US units,Like an ounce oz And liquid volume in ounces floz .Although metric units can be indicated simultaneously,Ruke g Milligrams mg And milliliters ml ,But American units are mandatory,And metric units are optional.
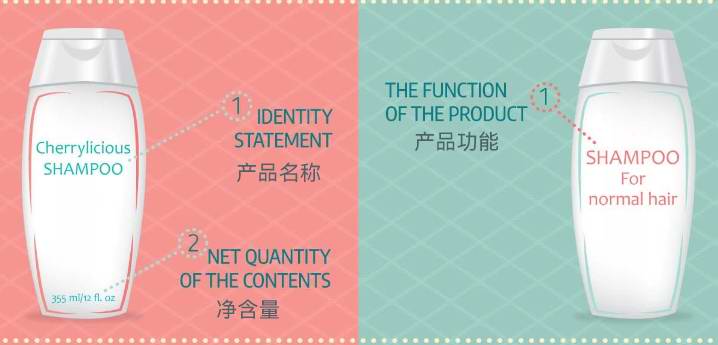
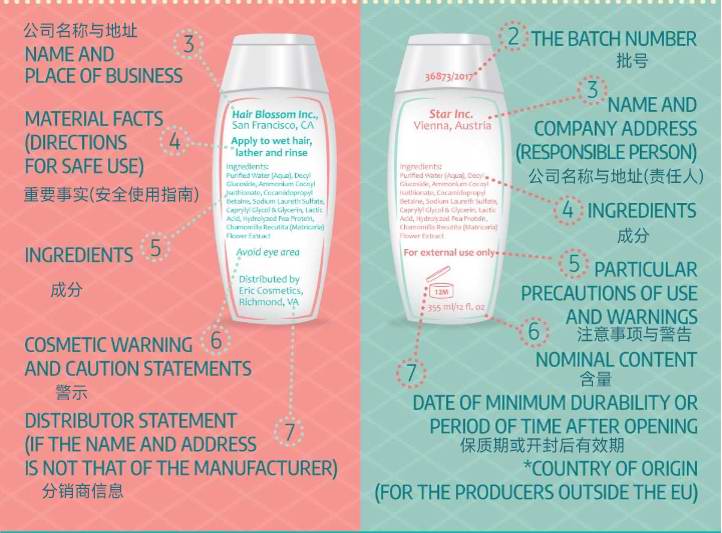
by comparison,The EU does not havePDPRequirements for the main display panel,Just indicate the product name on the main display panel.however,If the customer's product is to be sold in two or more countries,Or I hope to create a globally applicable label,Then the strictest requirements should be followed The standards of the countries with the most projects.
The back of the label is called the information side,Need to include company information Usage Guide Composition table Warning message and address of responsible person.Warning messages can be based on regulatory requirements for a certain component of the warning message,It can also be recommended by security assessment personnel.The EU has more requirements than the United States,Additional batch number and expiration date need to be indicated.For products manufactured in China,Whether in the US or EU markets,All need to be specified Made in China .