Product Compliance
filing/registration/consulting/training

This month, the Guangzhou Market Supervision Administration released Q&A on filing for ordinary cosmetics 62 issues Related to Q&A on filing for ordinary cosmetics Sixty three issues .
Guangzhou Municipal Supervision Q&A on filing for ordinary cosmetics 62 issues
answer according to Guidelines for submitting cosmetic safety assessment materials Regulations on the Classification of Cosmetics in China consult classification rule ,Claiming the efficacy of cosmetics User group Based on the classification of product dosage forms and other different dimensions,Taking into account different types of risk levels comprehensively,Increase the use of high-risk raw materials such as new or nano materials during the monitoring period Is it necessary to use instruments or tools in conjunction with other classification dimensions,And combine with the key focus of cosmetic safety assessment materials,Divide cosmetics into two categories.Cosmetic safety assessment materials are selected and submitted according to two categories and two situations,The first category of cosmetics requires submission of a cosmetic safety assessment report,On the premise of completing the cosmetic safety assessment report and ensuring that the quality management system of the registrant is in good operation, the second type of cosmetics,Scenario 1: Cosmetics can submit basic conclusions of cosmetic safety assessment and safety assessment materials corresponding to different risk points in the table below,Scenario 2: Cosmetics can submit basic conclusions of cosmetic safety assessment.The second type of cosmetics can also choose to directly submit a cosmetic safety assessment report.
Meanwhile, considering that the development of cosmetics requires a certain period of time,To avoid duplicate investment of R&D resources in enterprises, Announcement of the National Medical Products Administration on Issuing Several Measures to Optimize the Management of Cosmetic Safety Assessment 2024Year's Day50Number right2024year5month1The products that have been evaluated according to the original requirements have been set up recently1Transition period of the year,stay2025year5month1Recently,Cosmetic registrant The registrant can still submit documents that meet the requirements when applying for registration or filing Technical Guidelines for Cosmetic Safety Assessment 2021Annual edition Simplified version of security assessment report required.
Classification and Management of Cosmetic Safety Assessment Data
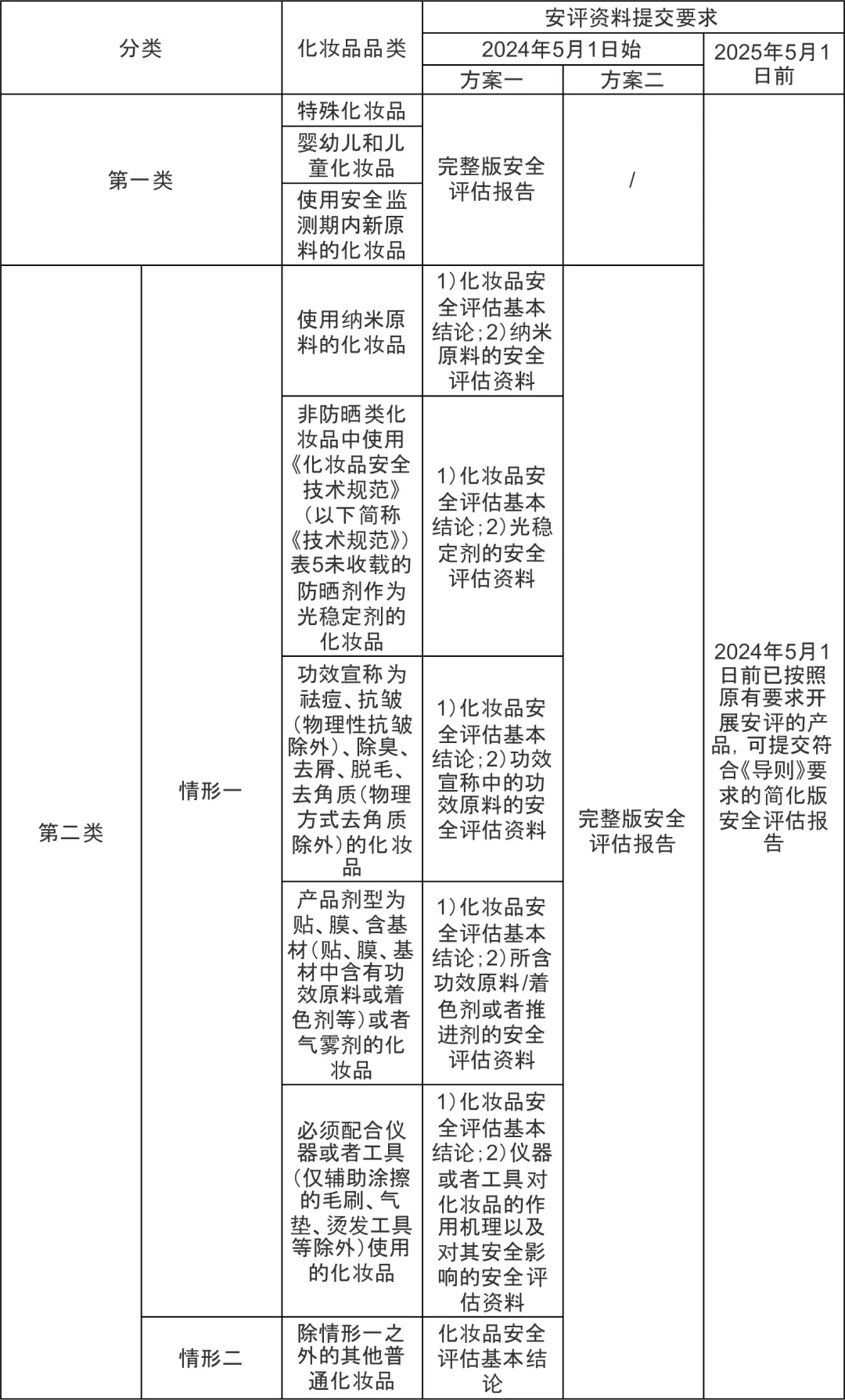
answer Guidelines for submitting cosmetic safety assessment materials Specify that the product formula contains nano raw materials,It should clearly include purity Crystal form Initial particle size distribution Quality specifications of raw materials for surface coating substances and other information,And based on the quality specifications of the raw material,Safety assessment of nano raw materials under formula usage,At the same time, it should be explained whether the toxicological testing methods used for evaluation are applicable to the detection of nanomaterials.
Due to the small particle size of nano raw materials,There is a high risk of inhalation exposure,Therefore, the use of nanomaterials in products that may pose a risk of inhalation exposure is not encouraged.
answer Guidelines for submitting cosmetic safety assessment materials The evaluation of essence and flavor has different requirements according to the filling method of essence and flavor in the formula Product Formula Table Standard Chinese name Only fill in the column Essence Raw material,Should be in accordance with Guidelines Evaluate essence according to the principles and requirements of,Or specify that the essence used in the product conforms to the International Daily Flavor Association IFRA Practice regulatory requirements or compliance with relevant regulations in China Essence national standards.Product Formula Table Standard Chinese name Fill in the column simultaneously Essence And specific flavor components in essence,Conduct safety assessments for each spice component.
Essence in children's cosmetics Plant essential oil or spice ingredients,Identification and evaluation of allergenic components are required.
answer Technical Guidelines for Filling in Cosmetic Formulas Prescription of cosmetic registrants The registrant or domestic responsible person shall be responsible for the quality of the selected raw materials,Raw materials not used as formula ingredients/Raw material composition,Can it not be reported in the product formula.
When evaluating raw materials,Simultaneously dealing with extremely small amounts of ingredients that are not reported as formula ingredients For example, extremely small amounts of ingredients added to cosmetic ingredients to ensure their quality Explain one by one and conduct a thorough safety assessment,Ensure that this type of ingredient does not affect the quality and safety of the raw materials.
answer Salicylic acid, also known as ortho hydroxybenzoic acid,It is a fat soluble organic acid. Safety technical specifications for cosmetics 2015Annual edition There are two regulations for the use of salicylic acid.A restricted component for use in cosmetics,See restricted components in cosmetics surface3 Number8 salicylic acid Regulations The maximum allowable concentration in resident and rinse skincare products is2.0%,The maximum allowable concentration in hair washing products is3.0%,None of them shall be used for3Products for children under the age of Except for shampoo .In this situation,The use of salicylic acid is not intended as a preservative,Its efficacy should be labeled on the product label.The second type is used as an approved preservative for cosmetics,See approved preservatives for cosmetics surface4 Number42 Salicylic acid and its salts Regulations The maximum allowable concentration in the product is the total amount0.5% Calculated by acid ,Not allowed for use3Products for children under the age of Except for shampoo .Salt refers to sodium potassium calcium magnesium Salts of ammonium and ethanolamines.
The sources of salicylic acid introduced into cosmetics may include3species One is salicylic acid esters,Under alkaline conditions, salicylic acid will be generated Secondly, plant extracts,The extract of white willow bark contains hydrosalicylic acid Ingredients such as salicylic acid The third is sugar beet alkaline salicylate,Synthesized from betaine and salicylic acid,Salicylic acid may be detected in its free form.
Cosmetic registrants should comprehensively analyze the overall formula ingredients,Scientific assessment of potential safety risk substances in products.Salicylic acid, whether used as a restricted component or as a preservative,None of them shall be used for3Products for children under the age of Except for shampoo ,Children's products should pay special attention to the safety risks of introducing salicylic acid into formula ingredients.
answer recent Administrative Judgment of Beijing Fourth Intermediate People's Court 2023 Beijing04End of line223Number for Removing dandruff and relieving itching The claimed judgment has sparked heated discussions in the industry.Dandruff often refers to a condition characterized by flaky scales on the scalp and hair,It usually causes skin itching spalling,Accompanied by mild inflammatory reactions.Cosmetic dandruff removal is generally achieved by removing some cells from the stratum corneum, thereby exerting the effect of exfoliation to improve the condition of dandruff or disrupt the environment required for the growth of Malassezia,Thereby reducing the production of dandruff,Pyrrolidone ethanolamine salt not only improves scalp dandruff but also reduces scalp immersion,To achieve the effect of relieving itching through the anti dandruff effect.in addition,The evidence provided by the involved merchant in the judgment shows that,Subjects using0.5%After using piroxicam ethanolamine salt dandruff removal shampoo,Significant itching relief Itching of the head caused by dandruff effect,Further objective verification of the scientific validity of the claim.
But it should be noted that,Cosmetics should be strictly distinguished from drugs,We should not exaggerate claims Relieve itching ,If it is targeted at mosquito bites Fungal infection eczema Itching caused by factors such as allergic dermatitis,It goes beyond the definition of cosmetics.The cosmetic registrant shall comply with the regulations issued by the National Medical Products Administration Classification rules and catalog for cosmetics Appendix1The efficacy claims in the classification directory21The efficacy, interpretation, and declaration guidelines of similar ordinary cosmetics,Ensuring scientificity Supported by evidence of reasonableness and adequacy,science Reasonably and legally label relevant claims.
Guangzhou Municipal Supervision Q&A on filing for ordinary cosmetics Sixty three issues
Special Requirements for Commissioned Production Filing Personnel
answer The client shall establish a cosmetic quality and safety responsibility system based on its organizational structure,For all departments involved in the entire quality assurance system Specific job positions Assign responsibilities to specific managers and operators, etc Authorization and implementation.Usually in the form of job responsibility documents or authorization letters,Written regulations on the legal representative of the enterprise(Or the main person in charge),Quality and Safety Manager,Record activity management Production quality management Responsibilities of the person in charge of key links such as product sales management and other cosmetic quality and safety related positions.Personnel in various positions of the enterprise shall fulfill their quality and safety responsibilities step by step according to their job responsibilities,Ensure the authenticity and traceability of relevant performance records.
answer 1.To clarify the format of the document/revise release Implementation process,Generally includes drafting or revising Auditing ratify grant train apply Recycling and destruction, etc.
2.Measures for regulating document management and control,Ensure that the formulated documents are effectively implemented and controlled,for example,Personnel in relevant positions should undergo training,Familiarize oneself with the content of relevant documents At the use site, the latest valid version is available,To avoid misuse of obsolete documents.
3.Regularly evaluate the formulated documents,Timely revise or abolish as necessary.
4.The quality management department should promptly recover any revisions made due to revisions Version change Abolish or invalidate documents.For previously controlled invalid documents,Enterprises should keep at least one copy,To meet the needs of traceability,Other invalid documents should be destroyed and destruction records should be kept.
answer Record keeping generally follows Who formed,Who saves it The principle of,Records should be retained by the implementers of relevant activities.therefore,The relationship between entrusted production and product production in entrusted production enterprises test Records related to factory release and other related matters,In principle, it should be kept at the entrusted production enterprise,The client can keep copies as needed.
The authenticity of the production records of the entrusted party by the client Integrity Responsibility for safety supervision.Various records generated by the commissioning party for quality management and supervision of commissioned production,Then it shall be kept by the commissioning party.
answer Implementation of commissioned production Dual release system.On the basis of the entrusted production enterprise completing the release of products from the factory, the commissioning party,Ensure that the product has passed inspection and relevant production and quality activity records have been reviewed and approved,Only then can it be released for listing.The specific review of release is generally carried out by the quality management department,The release documents should be reviewed and signed by the person in charge of the quality department Approved and signed by the person in charge of quality and safety or their authorized personnel.Enterprises should establish product release records,The record should include the product release time,Name of released product Batch number quantity Content of release inspection,And the review and approval conclusion.
answer 1.The cosmetic registrant shall establish and implement a product sales record system,And ensure the shipping documents of the products sold The sales records are consistent with the physical goods.Product sales records should at least include Product Name Registration number for ordinary cosmetics Usage period Net content quantity Sales date price,And the buyer's name Address and contact information, etc.
2.The cosmetic filing person shall establish and implement a return record system.The content of the return record should include Return unit Product Name Net content Usage period quantity Reasons for return and processing results, etc.
3.Cosmetic registrants should be equipped with the types of cosmetics they produce Institutions and personnel with appropriate numbers,Carry out adverse reaction monitoring work according to regulations,And form monitoring records.The record should at least include Reporter Information Information of individuals who have experienced adverse reactions Symptoms or signs Severity of adverse reactions Date of adverse reaction occurrence Date of discovery or knowledge of adverse reactions Adverse reaction report date Names of cosmetics used, etc.
4.The cosmetic filing person shall establish and implement a product recall management system,Implementing recall work in accordance with the law.The recall record should at least include Product Name Net content Usage period Recall quantity Actual recall quantity Reason for recall Recall time Processing results Report the situation to regulatory authorities, etc.