Product Compliance
filing/registration/consulting/training

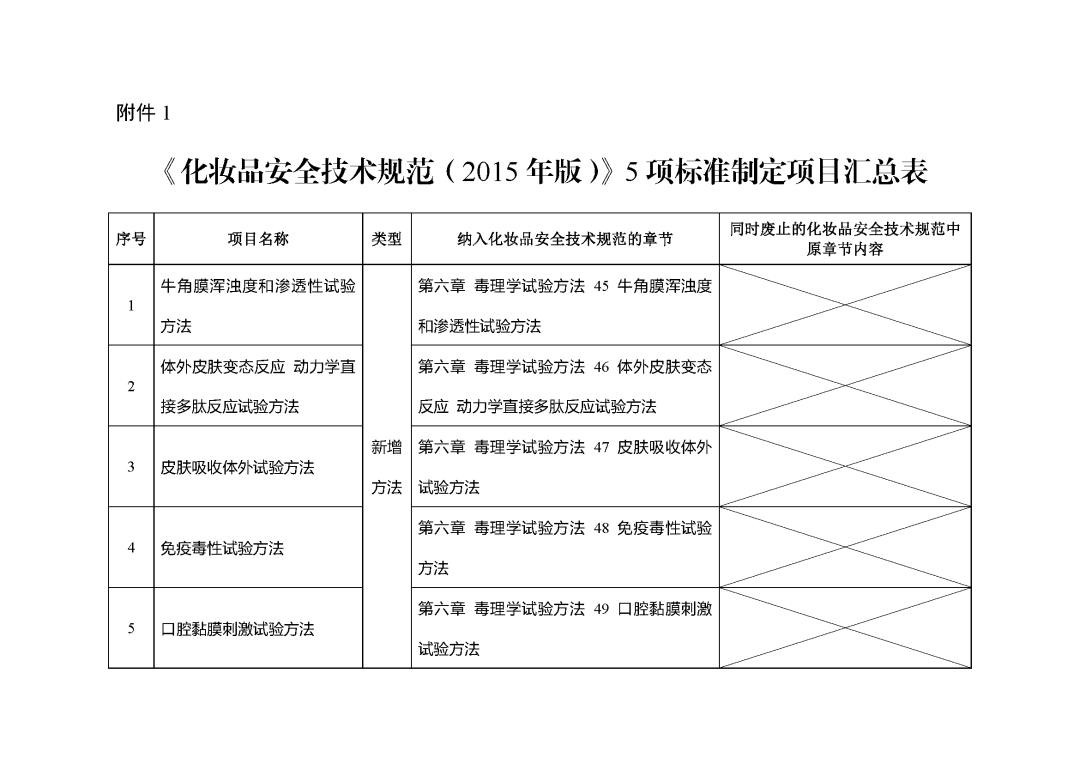
Five methods drafted by the National Medical Products Administration (NMPA), including the "Bovine Corneal Turbidity and Permeability Test Method," the "In Vitro Skin Allergy Kinetic Direct Peptide Reaction Test Method," the "In Vitro Skin Absorption Test Method," the "Immunotoxicity Test Method," and the "Oral Mucosal Irritation Test Method," have been reviewed and approved by the Directors' Meeting of the NMPA's Cosmetics Standardization Technical Committee. They are now published and incorporated into the corresponding chapters of the "Cosmetics Safety Technical Specifications (2015 Edition)" (see Appendix).
The above five methods are all new and will be implemented starting March 1, 2026. Prior to implementation, the use of these methods for cosmetics registration and filing-related testing is encouraged.
This announcement is hereby made.
Appendix:
1. Summary Table of the Five Standard Development Items for the "Safety Technical Specifications for Cosmetics (2015 Edition)"
2. Bovine Corneal Turbidity and Permeability Test Method
3. In Vitro Skin Allergy Kinetic Direct Peptide Reaction Test Method
4. In Vitro Skin Absorption Test Method
5. Immunotoxicity Test Method
6. Oral Mucosa Irritation Test Method
National Medical Products Administration
August 28, 2025
Contents of Annex 1 (Please read the original text for other information):