Product Compliance
filing/registration/consulting/training

In recent years, exosome Rapidly gaining popularity in the fields of skincare and regenerative medicine.various Exocrine body essence Exocrine facial mask emerge in an endless stream,Promote everything from moisturizing and repairing to anti-aging and anti-aging. But at the regulatory level,The identity of this type of product is not ambiguous They may be cosmetics,It could also be a drug.
Health Canada Health Canada Latest Announcement,This clarifies the classification principles and regulatory requirements for such products.
1. Why is classification important
In Canada, Food and Drug Law Food and Drugs Act, FDA Prescribed drugs Drug cosmetics Cosmetic Definition of Waiting. Product Features purpose Publicity,Decided its regulatory identity
classification | regulatory requirements |
medicine | Must undergo clinical validation Approved NOC DIN To be listed |
cosmetics | Registration required Cosmetic Notification ,But it cannot be promoted with therapeutic or medical functions |
2. Regulatory principles for extracellular vesicle products
Extracellular vesicle substances are not natural medicine ,But they may be affected by their effectiveness Recognized as a drug based on its usage method.
The main considerations include
1) Product claims Representation
Medicine : If promoted Treat skin diseases Repair cell Reverse aging Waiting for medical efficacy,Will be recognized as a drug.
Cosmetics :Only limited to Improve skin texture moisturizing brighten Waiting for beauty benefits,Not involving treatment.
2) No Observed Adverse Effect Level Level of Action
CosmeticsOnly applies to the surface skin,The effect should be Mild and brief
If the product needs to pass through microneedle injection Promote infiltration through other means,Or it must be absorbed to exert a systemic effect,So it belongs to the category of drugs.
3) Composition Composition
Medicine : Containing substances that can affect bodily functions Ingredients with pharmacological activity.
Cosmetics : Not possessing therapeutic or pharmacological activity.
3. Cosmetics vs medicine Example comparison
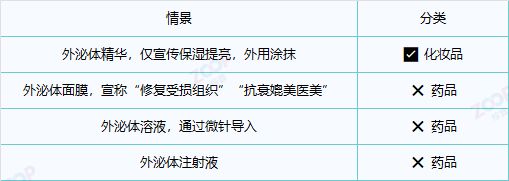
4. Regulatory requirements in Canada
1) Approval is required before listing NOC DIN
2) Clinical research stage requires obtaining No Objection Letter (NOL)
3) Unapproved products pose a risk of infection and transmission Unverified therapeutic risks
Cosmetics products
1) Enterprises need to do so after the first sale10Filing within days
2) Filing ≠ approval,It does not necessarily mean that the product is compliant
3) Enterprises need to ensure compliance on their own Food and Drug Law and Cosmetics Regulations
5. Ministry of HealthReminder and Public Participation
1) Unauthorized extracellular vesicle products may have false efficacy claims potential toxicity Risk of infectious diseases
2) The public can file complaints or report adverse events through the Health Canada platform
Public consultation period
Since the announcement was released 75 Within the day,enterprise Practitioners and consumers can submit opinions or scientific data,For policy improvement reference.
6. Summary
● Say Skincare and Beauty ,It can also be registered as a cosmetic product
● Say Treatment and repair ,That's drug management
For enterprises,How to position and promote products,It will directly determine its regulatory identity and compliance risks.
For consumers,bought Exocrine body essence What exactly is it skincare products still medicine ,The future may become increasingly clear.
Services provided by Zoop
◆ General cosmetics, natural health products, and over-the-counter drug notifications
◆ Product formula and labeling review
◆ Product safety certification testing and safety assessment services
◆ Cosmetics classification services
◆ Canadian cosmetics regulatory compliance training
◆ Other customized services