Product Compliance
filing/registration/consulting/training

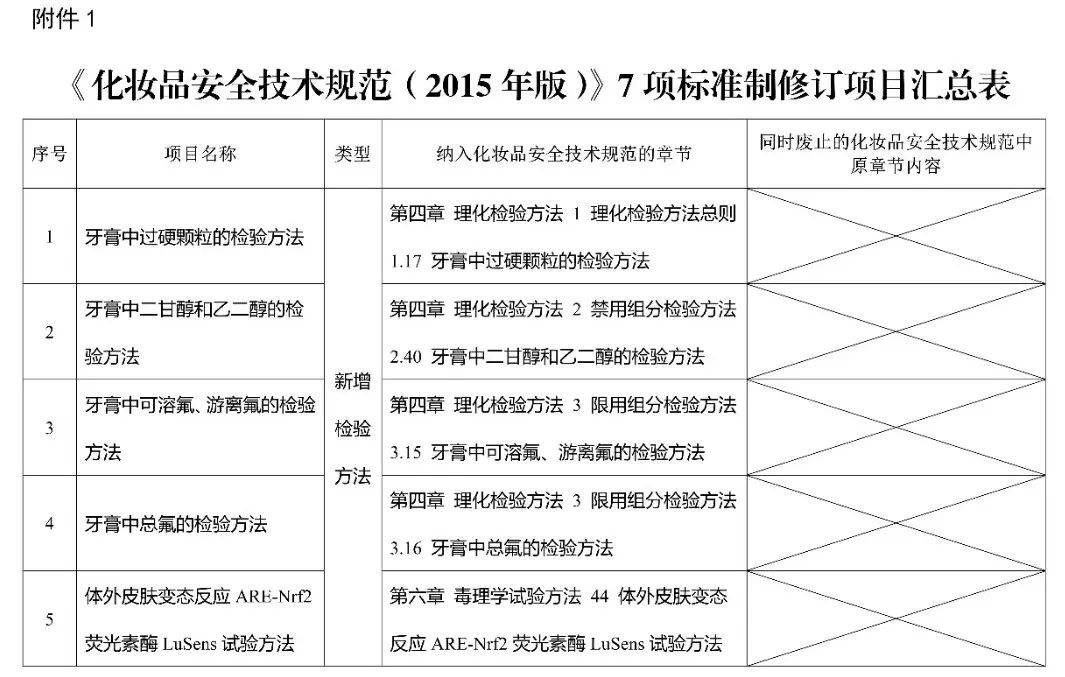
The National Medical Products Administration has organized the drafting of seven methods, including the "Inspection Method for Hard Particles in Toothpaste", which have been reviewed and approved by the Chairman's Meeting of the Cosmetics Standardization Technical Committee of the National Medical Products Administration. They are now released and included in the corresponding chapters of the "Cosmetics Safety Technical Specifications (2015 Edition)" (see Annex 1).
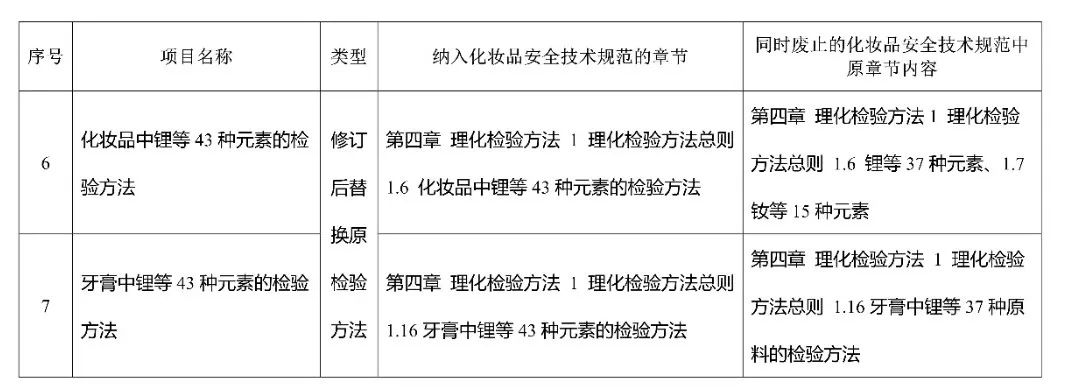
The inspection methods for hard particles in toothpaste, diethylene glycol and ethylene glycol in toothpaste, soluble and free fluoride in toothpaste, total fluoride in toothpaste, and in vitro skin allergy ARE-Nrf2 luciferase LuSens test method are newly added methods (see Appendix 2-6). The "Methods for Testing 43 Elements including Lithium in Cosmetics" and "Methods for Testing 43 Elements including Lithium in Toothpaste" are revised testing methods (see attachments 7-8), replacing the original testing methods in the "Cosmetic Safety Technical Specifications (2015 edition)".
The above method will be implemented from March 1, 2026. Before implementing the method, encourage the use of the above methods for cosmetics registration and filing related inspections.
This is to inform you.
Enclosure:
1.Summary Table of Revision Projects for 7 Standards of Cosmetic Safety Technical Specifications (2015 Edition)
2.Test method for hard particles in toothpaste
3.Method for Testing Diethylene Glycol and Ethylene Glycol in Toothpaste
4.Test method for soluble and free fluoride in toothpaste
5.Test method for total fluoride in toothpaste
6.In vitro skin allergy ARE-Nrf2 luciferase LuSens assay method
7.Testing method for 43 elements including lithium in cosmetics
8.Testing method for 43 elements including lithium in toothpaste
SFDA
May 6, 2025
Attachment 1 Content:
Please scan the code to view the remaining content:
