Product Compliance
filing/registration/consulting/training

introduction
The China National Institute for Food and Drug Control successfully held an event in Shenzhen in early September Cosmetic Safety Assessment Technology Training Course .Zongpu has sorted out some hot issues,Share the requirements for safety evaluation in current registration and filing, as well as industry trends in October.
It should be emphasized that,The answers to relevant questions should be based on laws and regulations, as well as the opinions of the reviewing teacher.If you have any questions or need more information,Welcome to communicate with our team at any time.
This article is the second content,MainlyStability evaluation Compatibility of packaging materials Preservatives Challenge and Other Related Issues.
Stability evaluation
A The registrant can submit a registration and filing application after the acceleration test is completed,Long term test report retained by the enterprise for future reference.
A The purpose of evaluating the stability of cosmetics is to examine them at a certain temperature The law of time variation under humidity and other conditions,Influence factor test Accelerated testing The long-term testing time is determined by the registrant based on the characteristics of the cosmetics Determination of packaging materials and other factors.Multiple experimental time points need to be set up to study the quality changes of cosmetics,The setting of time points should be based onProduct form characteristicsandStability trendevaluateRequest to wait to set up.
A Approximate formula system Cosmetics with the same packaging material,Stability evaluation can be carried out based on existing data and experimental data,But the reasons need to be explained,describe the circumstances.
Compatibility assessment of packaging materials
A The compatibility test between cosmetics and their packaging materials is forTechnical research conducted to investigate the interaction between cosmetics and packaging materials.Due to the variety of materials used in packaging Various shapes and a wide variety of packaged cosmetics,Based on the principles of risk management,Based on reference to drug and food related standards,Formulate based on the current situation of the cosmetics industry Compatibility Technical Guidelines .Cosmetic registrant After evaluating the packaging materials of the product, the registrant,You can refer to this guide to conduct compatibility testing,Thus strengthening the supervision and management of cosmetics,Further improve the safety of cosmetics use,Guide the industry to pay attention to the potential risks arising from packaging materials in cosmetics.
A When choosing packaging materials for cosmetics,Cosmetic registrant The registrant can determine the composition of the cosmetics based on their ingredients Assess the compatibility between cosmetics and packaging materials based on actual risk levels and other factors.
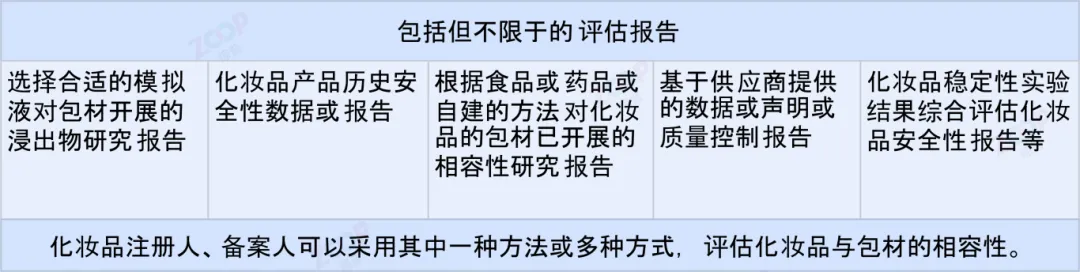
If the cosmetics registrant The registrant is unable to provide the above-mentioned safety assessment materials or discovers that cosmetics interact with their packaging materials and affect the quality of cosmetics When safety is affected,Reference should be made Compatibility Technical Guidelines Conduct relevant research.
A Approximate formula system for cosmetics When the packaging material of the container or carrier in direct contact with the content is the same and the source is consistent,Cosmetic registrant The registrant may conduct compatibility evaluation based on existing information and experimental data,But the reasons need to be explained,describe the circumstances.
Preservative Challenge
A Guidelines for Testing and Evaluation Techniques of Cosmetics Anti corrosion Challenge hereinafter referred to as Technical Guidelines recommendCMCCStrain as test strain,Mainly based onThe simplicity of the purchasing process and the accessibility of bacterial strains.ATCCAlthough the strain originated from the Global Center for Biological Resources,But the price is high,The purchasing process is complex.Users are making purchasesATCCStrain time,Need to provide a commitment letter included application Unit Introduction Biosafety Certificate Multiple materials including product transfer agreement and business license,And accept the supervision and inspection of Chinese customs.These requirements apply to the majority of cosmetics registrants in China For the registrant,Undoubtedly, it has increased the difficulty significantly,Resulting in many cosmetics registrants Difficulty in obtaining the record holder.in addition,Comprehensive reminderATCCThe transportation cycle of bacterial strains is long,May bring uncertainty to the experiment.
by comparison,CMCCStrain as the standard strain in China,Relatively cheap in price and easy to obtain,At the same time, its purchasing process is relatively simple,As a cosmetic registrant The registrant saved a lot of time and costs,More suitable for anti-corrosion challenge testing.
A book Technical Guidelines AsNon mandatory reference documents,Its main purpose is for cosmetic registrants The registrant provides guidance and suggestions when conducting relevant tests.Cosmetic registrant Filing personCan be tailored to one's actual needs and situation,Select industry standards international standard asISO 11930 Or self built methods,Comprehensively evaluate the effectiveness of the product's anti-corrosion system.
A Mixed bacterial inoculation may lead to differences in experimental results compared to single bacterial inoculation,Increasing the complexity and uncertainty of result evaluation. Technical Guidelines The method of mixed infection inoculation has not been adopted in China,Recommended practices that can follow international standards.
A For products with the same anti-corrosion system and similar formulas,The effectiveness of the product's anti-corrosion system can be evaluated by referring to existing materials and experimental data,Formulate an anti-corrosion challenge evaluation report,And submit explanatory materials for its anti-corrosion system and formula system.
Other related issues
A 1 Guidelines for Registration and Filing Inspection of Cosmetics 2019Year's Day72number
Cosmetics companies should comply with regulations Mandatory national standards Standard requirements,Select inspection and testing institutions with corresponding inspection capabilities.Before conducting cosmetic registration and filing inspection work, inspection and testing institutions,Should obtain the qualification certification of inspection and testing institutions in the cosmetics field CMA ,And the scope of ability to obtain qualification certification can meet the needs of cosmetics registration and filing inspection work.
2 Regulations on the Management of Registration and Filing Materials for New Cosmetics Ingredients 2021Year's Day31Announcement No
Toxicological test report and anti-corrosion Sunscreen Spot removal and whitening Inspection report on anti hair loss efficacy evaluation and other related items,Should be certified by inspection and testing institutions that have obtained qualifications in the field of cosmetics CMA Or China National Accreditation Service for Conformity Assessment CNAS approval,Or comply with internationally recognized good clinical practice standards GCP Or good laboratory operating procedures GLP Issued by qualified or recognized inspection agencies.
3 Technical Guidelines for Safety Assessment of Cosmetics 2021Year's Day51Announcement No
The reference materials cited in the safety assessment of cosmetics should be technical reports published in full text format announcement Professional books or academic papers,And data or risk assessment materials released by international authoritative institutions, etc When applying unpublished research results,Zongpu reminds that consent from the data owner is required,And analyze the scientificity of the results accuracy Authenticity and reliability, etc.
A Fully consider the lack of toxicological data for some raw materials that are currently highly sought after and of concern in the industry The difficulty of gaps in security assessment data still exists,according to Guidelines for Submitting Cosmetic Safety Assessment Data Key points of self-examination in,Clarify the applicable principles for safety testing of cosmetic final products.Registered person The registrant or safety assessor shall conduct sufficient research on the data types and relevant toxicological data of the raw material evaluation,If it is determined by oneself that it cannot be adopted Guidelines for the Use of Cosmetics Raw Materials Data Evaluate any data type in the,When conducting a complete assessment according to the risk assessment procedure,It is indeed impossible to obtain relevant data on some toxicological endpoints of the raw materials,At the same time, the content of raw materials in the formula is relatively low,Does not have special effects such as spot removal, whitening, or hair loss prevention,And the number of raw materials in the above situation shall not exceed the total number of ingredients in the formula10%,At this point, final product safety testing can be conducted,Comprehensive evaluation and analysis of the safety of the final product.
Due to significant differences in the structure and function of different raw materials,Registered person, filer,Alternatively, safety assessors may need to consider the structural characteristics of the raw materials Physical and chemical properties product type Factors such as the product's functional area and usage method have an impact on Low content Conduct a comprehensive definition.
A may not.The human safety test should be conducted first before the human efficacy test.
①Fill in the propellant separately Total Content100%
②Convert formula quantity and safety limit
③Whether to use nano materials Is it a children's product, etc
④If the risk of lung inhalation cannot be ruled out,Need to provide safety assessment data on inhalation toxicity
The services provided by Zoop
◆ Full process declaration service for special certificates
◆ Imported cosmetics declaration service
◆ General makeup/Toothpaste filing service
◆ New raw material filing service
◆ Raw material safety assessment service
◆ Raw material submission code application service
◆ Cosmetics export compliance services
◆ Customized chemical specification training