Product Compliance
filing/registration/consulting/training

lead word
The China National Institute for Food and Drug Control held an event in Shenzhen in September Cosmetic Safety Assessment Technology Training Course .Zongpu has sorted out some hot issues,Share the requirements for safety evaluation in current registration and filing, as well as industry trends in October.
It should be emphasized that,The answers to relevant questions should be based on laws and regulations, as well as the opinions of the reviewing teacher.If you have any questions or need more information,Welcome to communicate with Zongpu at any time.
This article is the first content,The main issue is the use of safety evaluation raw material dataTTCMethod related issues.
Issues with the use of safety assessment raw material data
A WHO FAOAnd other international authoritative organizations,Regarding food Food additives and chemical use, etc,Publish daily allowable intake ADI Daily tolerated dose TDI Reference dose RfD Substances generally considered safe GRAS And spice standards, etc,We will also summarize the toxicological data of chemicals,Provide toxicological endpoint data.Regulatory authorities in China and other countries will also release information on the safe usage of cosmetic ingredients.During the cosmetics evaluation process,Evaluation data or conclusions from these authoritative institutions can be used,But it is necessary to analyze the relevant information,There is sufficient data support,And in compliance with relevant regulations on cosmetics in China,Relevant conclusions can be adopted.When the evaluation results of different authoritative institutions are inconsistent,Based on the reliability and correlation of the data,Scientifically and reasonably adopt relevant evaluation conclusions.If there is a lack of local toxicity data,It should be combined with the location and usage method of the product,Conduct an assessment of local toxicity.
A The experimental data can be combined with the product itself to conduct an evaluation.
A Assessment data or conclusions published by international authoritative cosmetic safety assessment agencies, including but not limited to the above-mentioned assessment agencies, may be used.Cosmetic registrant The registrant shall fulfill the main responsibility of the enterprise,When applying internationally authoritative cosmetic safety assessment data,The latest evaluation report shall prevail,And analyze the relevant information,Subject to compliance with relevant regulations on cosmetics in China,Relevant evaluation conclusions can be adopted.
A Raw material usage in resident products for the eyes,Can be used Raw material information The usage amount of this raw material in the resident products for the eyes in China,No need to evaluate eye irritation If none,According to principle two of use,The amount of this material that can be used for the whole body skin or trunk area, face or lips,Comprehensive reminder to separately evaluate eye irritation.For more questions and detailed answers, please refer to the document released by the National People's Procuratorate Information on the use of raw materials for products already on the market Q&A
A Will update,And consider including it in the registered product data.
Q The usage of raw materials for products already on the market exceeds Use information The quantity in the middle,How to conduct a security assessment
A Use information The quantity in is a reference value and not a limit value,The usage of raw materials exceeds Use information The quantity in should be according to Technical Guidelines for Safety Assessment of Cosmetics 2021Year Edition Conduct security assessments or follow Guidelines for the Use of Cosmetic Raw Material Data Use other raw material data types.
A 1 Same catalog number 2 source Consistent specifications, etc,The above two points can be used as references.
A Can refer to the principle of new raw materials,New raw materials to be registered and filed for further processing on the basis of raw materials,It should be fully demonstrated that the raw materials used in the production of new cosmetic ingredients are consistent with the food ingredients stated in the data Requirement as above ,And the processing method should be basically consistent with the food consumption processing method If only water is extracted Steaming and cooking, etc ,If necessary, analyze the changes in composition and enrichment situation.
A No,,Different consumption histories have different sources.
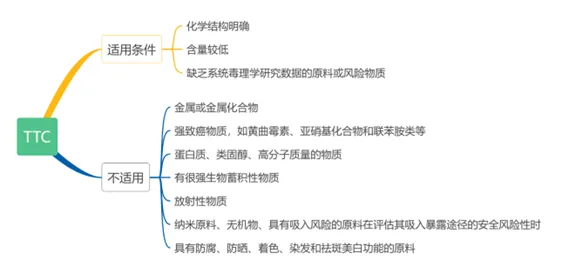
Toxicological concern thresholdTTC
A Technical Guidelines for Safety Assessment of Cosmetics 2021Year Edition and Toxicological concern threshold TTC Method Application Technical Guidelines regulations For clear chemical structure,And it does not contain raw materials or risk substances with serious mutagenic warning structures,When the content is low and there is a lack of systematic toxicology research data,Can refer to the use of toxicology attention threshold TTC Method for evaluation.
A 1.Experimental testing Analyze based on mutagenicity test data
2. ration Structure performance relationship prediction,At least two complementary internationally recognized forecasting methods should be used,A method should be based on expert knowledge rules,Another method should be based on statistics.The prediction methods for expert knowledge rules include QSAR TooIbox(OECD) Toxtree(EU Joint Research CenterJRC) OncoLogicTM(US Environmental Protection AgencyEPAandOECD)etc..The statistical prediction methods include The US-EPA'sToxicity Estimation Software Tool(T.E.S.T. ,EPA) Lazar(German Federal Institute for Risk AssessmentBfR)etc..
Some prediction methods may include multiple sub models,asVEGA(Italian Institute of Scientific Health ResearchIRCCS)Mutagenic model.in addition,There are also some business model prediction methods that can be chosen to use.
A Plant extracts have complex components and variations between batches,There are difficulties in identification and analysis techniques,Therefore, under certain conditions,Some unknown components in the raw materials can be usedTTCMethod for evaluation.
Through laboratory testing or literature search,Collect the sources of plants Preparation process chemical composition Characteristic components Physical and chemical properties quality standard Impurities and other information,Identify as many single or large categories of components as possible,Especially the main chemical components and characteristic components, etc,The component content can be calculated based on the category of components.Comprehensive reminder to add necessary solvents or stabilizers during the production process preservative Antioxidants and other external substances,The required ingredient content should not be less than50 %.Afterwards, based on the content of each component in the plant raw materials,Chemical Structure and Safety Information,Design a comprehensive strategy for security assessment.
The services provided by Zoop
◆ Full process declaration service for special certificates
◆ Imported cosmetics declaration service
◆ General makeup/Toothpaste filing service
◆ New raw material filing service
◆ Raw material safety assessment service
◆ Raw material submission code application service
◆ Cosmetics export compliance services
◆ Customized chemical specification training