Product Compliance
filing/registration/consulting/training

To promote the implementation of the cosmetics safety assessment system,according to Announcement of the National Medical Products Administration on Issuing Several Measures to Optimize the Safety Assessment and Management of Cosmetics 2024Year's Day50number ,The China National Institute for Food and Drug Control has formulated and released Guidelines for Submitting Cosmetic Safety Assessment Data and Guidelines for the Use of Cosmetic Raw Material Data .These two guidelines further refine the submission requirements for safety assessment data and optimize the data submission process meanwhile,Standardized the process of raw material safety assessment,Clarified the types of evaluation data.
zoop Regarding here Guidelines for Submitting Cosmetic Safety Assessment Data and Guidelines for the Use of Cosmetic Raw Material Data Carefully organized,Intended for filers/The registrant provides guidance and reference during the product registration and filing process.
Requirements for submitting classified safety assessment reports
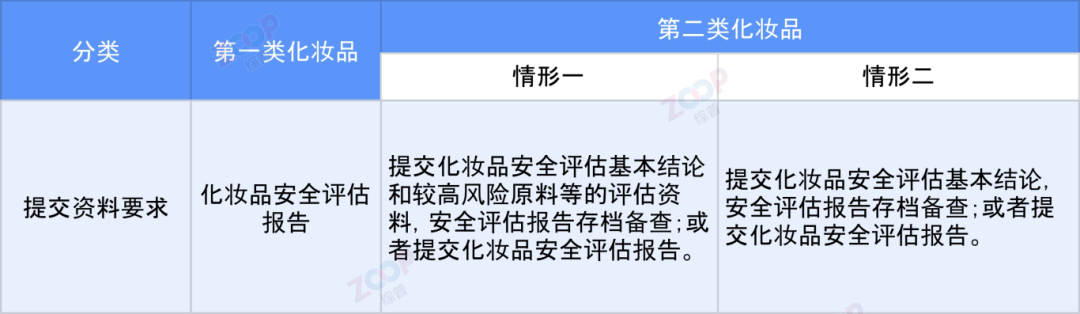
Submission Guide reference Classification rules and catalog for cosmetics ,Based on risk management principles,Taking into account the risks associated with different types of cosmetics and the key focus areas in cosmetic safety assessment materials,Claiming the efficacy of cosmetics Target audience Product dosage form Whether to use new or nano materials during the monitoring period Whether it is necessary to use instruments or tools can be classified into two categories based on classification dimensions.

Guidelines for the Use of Cosmetic Raw Material Data
The guide specifies the registrant of cosmetics The registrant may use when conducting safety assessments7Types of raw material data Usage requirements and required proof materials.
That's all for this article7Sort out and provide examples of various data types.
Raw material data available for safety evaluationfrom3The class has been extended to7class,They are respectively
one Technical specifications for cosmetic safety Hereinafter referred to as technical specifications Restricted components in Approved preservatives Approved sunscreen Approved colorants and hair dyes.
two Assessment conclusions published by international authoritative cosmetic safety assessment agencies.
three world health organization WHO Food and Agriculture Organization of the United Nations FAO Waiting for the safety limits or conclusions announced by authoritative institutions.
four Information on the use of raw materials for listed products released by regulatory authorities.
five raw material3Annual usage history.
six Safe consumption history.
seven Polymer with stable structure and properties Excluding raw materials with high biological activity .
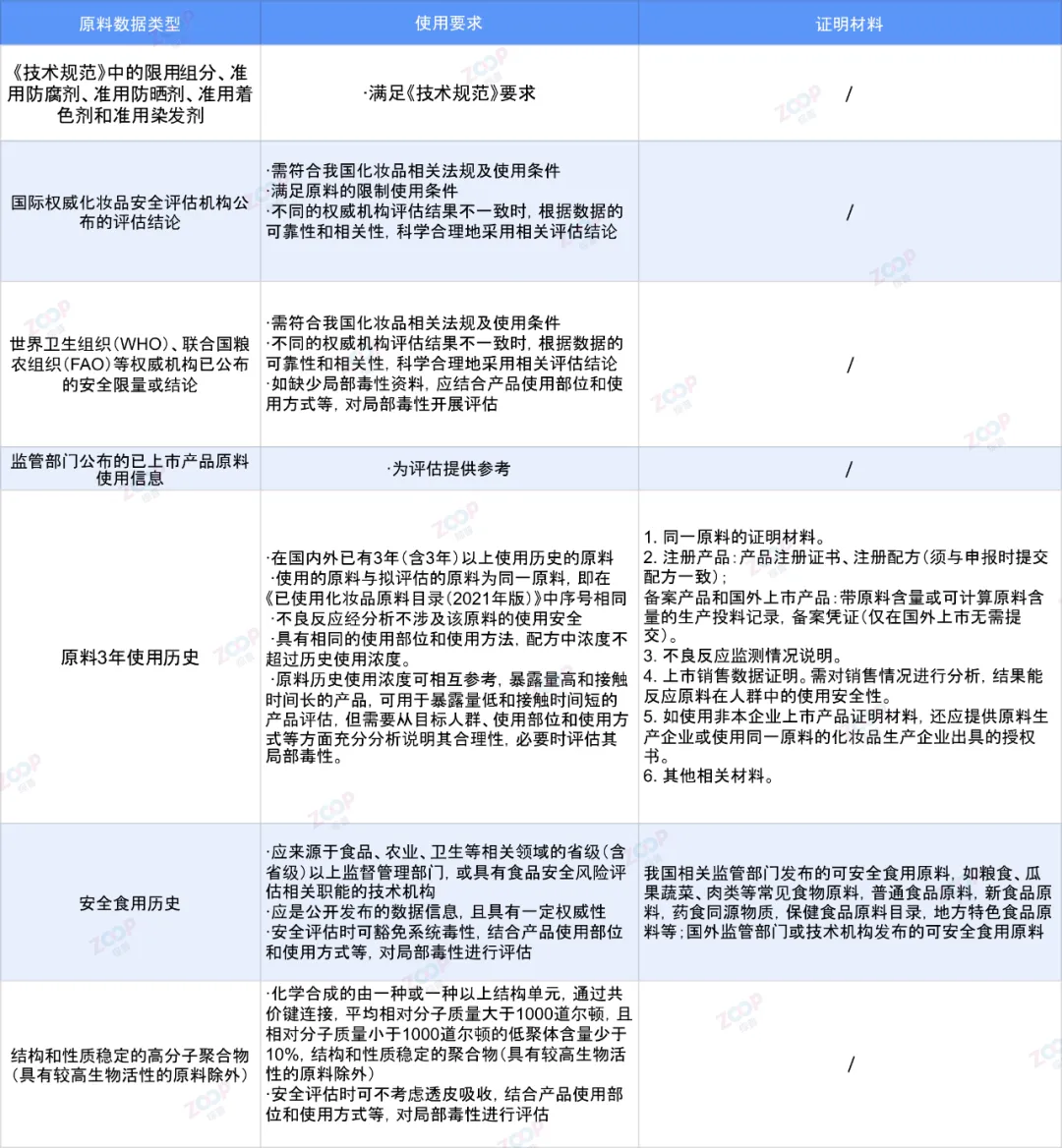
It should be noted that the raw materials used as freckle whitening agents and anti hair loss agents,Cannot use the raw material usage information of listed products published by regulatory authorities.Spot removing and whitening agents cannot use raw materials3Annual use history as evaluation evidence When used for the same purpose,Anti hair loss agents can use raw materials3Annual use history as evaluation evidence.
Zoop closely monitors the latest developments in the comprehensive safety assessment of cosmetics,Share security assessment details,Attentive service to every customer.Our team has multiple experienced safety assessment researchers,Proficient in the regulations and technical requirements of the cosmetics industry,Being able to provide timely services to customers Professional compliance services for products and raw materials.With years of industry experience and a deep understanding of regulations,We provide comprehensive protection for your cosmetics registration and filing,Assist in the smooth launch of your product.