Product Compliance
filing/registration/consulting/training

Definition of Imported Cosmetics CosmeticsThe last contact with the contentThe process is inabroadCompleted as an imported product,In Taiwan, China, China Reference import product management completed in Hong Kong and Macau regions.
Management Measures for Registration and Filing of Cosmetics Article 61
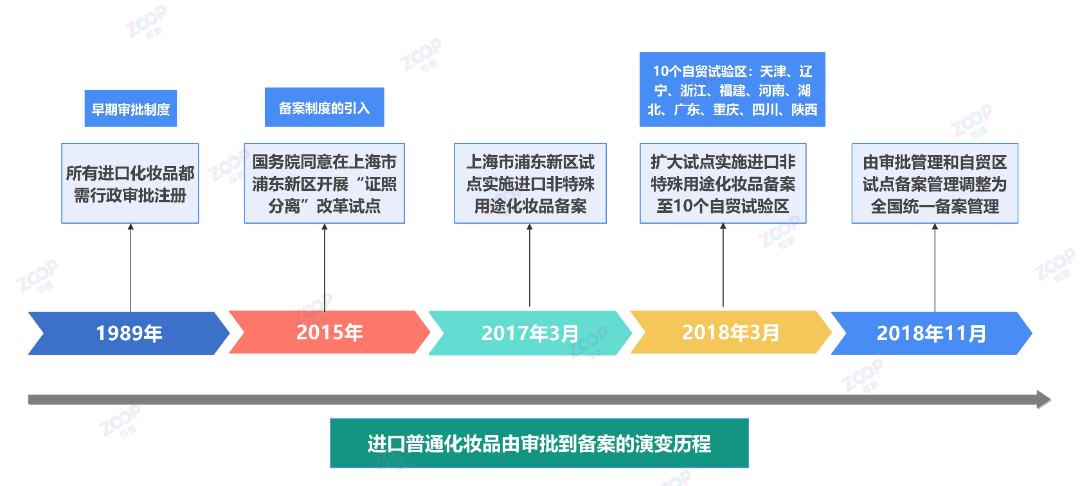
The registration and filing system for imported ordinary cosmetics in China has undergone the following major evolutionary history
Early approval system early,All imported cosmeticsAll of them need to go through the China Food and Drug Administration CFDA,presentNMPA The administrative approval registration process,This includes strict scrutiny of product ingredients and safety.
1989Annual release Regulations on the Supervision of Cosmetics Hygiene Article 15,First time imported cosmetics,The importing unit must provide the instruction manual for the cosmetics quality standard Relevant information and samples on inspection methods, as well as the exporting country region Proof of Approval for Production,Approved by the health administrative department of the State Council,Only then can an import contract be signed.
Introduction of filing system With the development of the Chinese cosmetics market and its opening up to the outside world,China begins to implement a cosmetics filing system,Simplified the admission process for imported ordinary cosmetics.
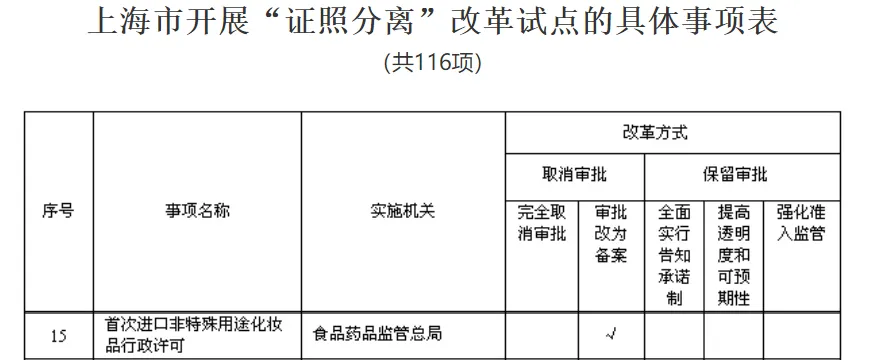
2015The State Council agreed to carry out the project in Pudong New Area, Shanghai in Separation of certificates and licenses Reform pilot,The administrative licensing matters for the first import of non special purpose cosmetics shall be handled byChange approval to filing.

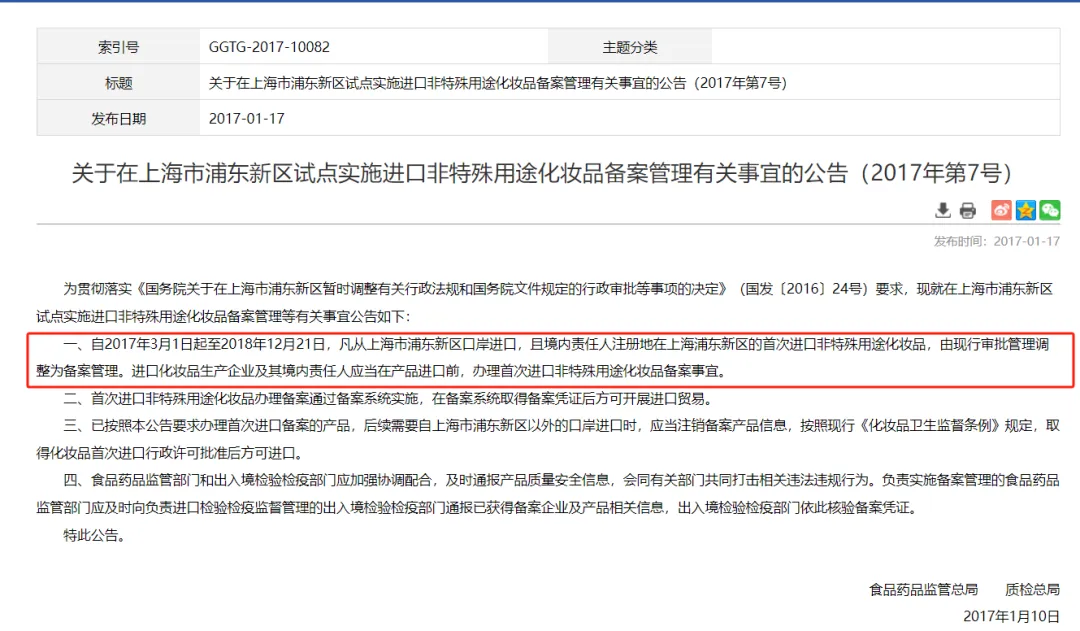
2017year3month,Pilot project in Pudong New Area, ShanghaiimplementationRegistration of imported non special purpose cosmetics.
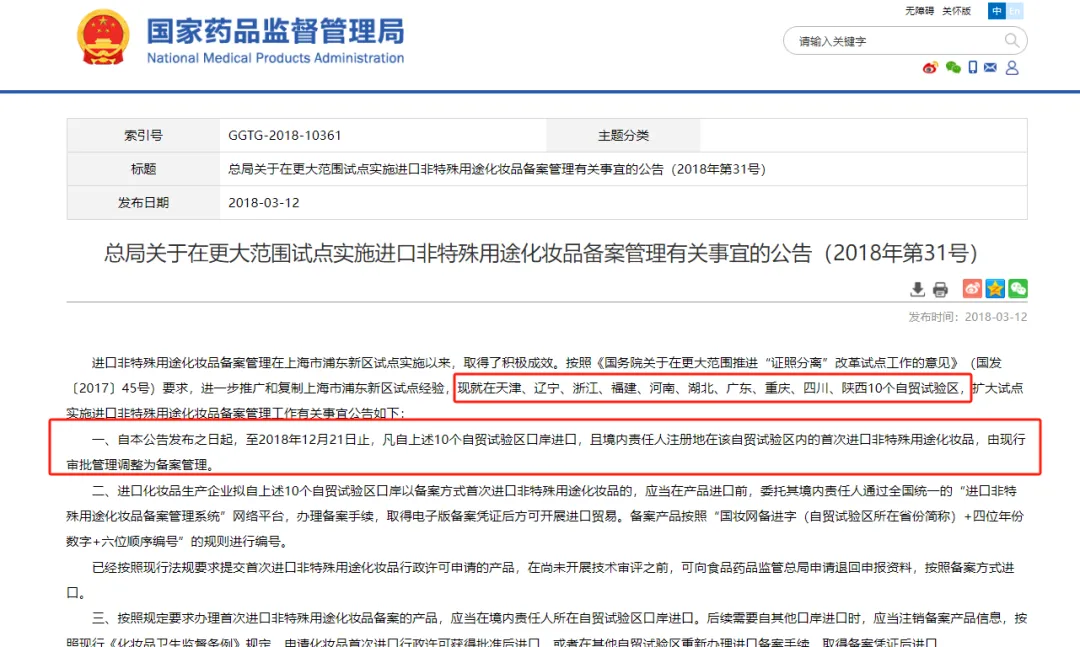
2018year3month,Expand pilot implementation of import non special purpose cosmetics registration to10A pilot free trade zone Tianjin Liaoning Zhejiang Fujian Henan Hubei Province Guangdong Chongqing Sichuan Shaanxi .reference Management Procedure for Filing of Imported Non Special Purpose Cosmetics in Pudong New Area, Shanghai provisional Implementation of relevant requirements.
2018year11month,Adjusted from approval management and pilot registration management in free trade zones toNational unified filing management,At the same time, administrative license applications for the first import of non special purpose cosmetics will no longer be accepted.Already carried out11The filing of a pilot free trade zone shall be handled by the provincial food and drug supervision and administration department where it is located,Other provinces outside area city Within the administrative region,Implement online submission of electronic information,Filing with the National Food and Drug Administration.
The current registration and filing management system for imported cosmetics
2020year6month,The State Council has announced Regulations on the Supervision and Administration of Cosmetics ,And in2021year1month1Effective from [date].It is explicitly stipulated that imported special cosmetics must be registered with the drug regulatory department of the State Council,Import ordinary cosmetics and register with the drug regulatory department of the State Council.And the National Medical Products Administration can entrust provinces with corresponding capabilities Autonomous Region The drug supervision and administration departments of municipalities directly under the central government implement the filing management of imported ordinary cosmetics.
Comprehensive reminder,The registration and review department for imported ordinary cosmetics shall review the responsible persons within the country Filing person There are differences depending on the location.In addition to the aforementioned initiatives that have already been carried out11The registration of a pilot free trade zone shall be handled by the province where it is located city The provincial drug supervision and management department undertakes the filing and review of imported ordinary cosmetics, in addition to,Shandong Beijing Hunan Hainan Jiangsu Jilin and other provinces city We have also successively undertaken the filing and review of imported ordinary cosmetics,And some provinces that have not delegated their review authority city For example, Jiangxi Gansu Province,Xizang, etc,At present, imported ordinary cosmetics are still subject to review by the National Administration.
