Product Compliance
filing/registration/consulting/training

new primary material state report separate read remove spot beautiful white × peaceful complete eat use experience history
New raw materials with spot removing and whitening functions,Can be adjusted according to the situation5Declaration Classification of new raw materials for cosmetics situation1 The first domestically and internationally used product with anti-corrosion properties Sunscreen color dye the hair Spot removal and whitening Anti-Hair Loss acne treatment anti-wrinkle Excluding physical wrinkle resistance Dandruff Deodorizing function and other newly used cosmetic ingredients with high biological activity for the first time both domestically and internationally. situation5 haveSafe consumption historyNew raw materials for cosmetics The parts used for raw materials should be consistent with the edible parts . 2024year5the moon,Published by the Central Inspection Institute Guidelines for Historical Research and Determination of Safe Consumption of New Cosmetics Ingredients trial Draft for soliciting opinions ,After a year, Guidelines for Historical Research and Determination of Safe Consumption of New Cosmetics Ingredients trial Hereinafter referred to as guide Formal implementation trial.The official version of the guidelines is based on the draft for soliciting opinions,Some new content has been proposed again. |
one Safe consumption history≠situation5
Compare the draft for soliciting opinions with the official draft,It can be found that,The official draft no longer emphasizes situation5 ,But rather Corresponding situation Replaced the original draft for soliciting opinions situation5 .
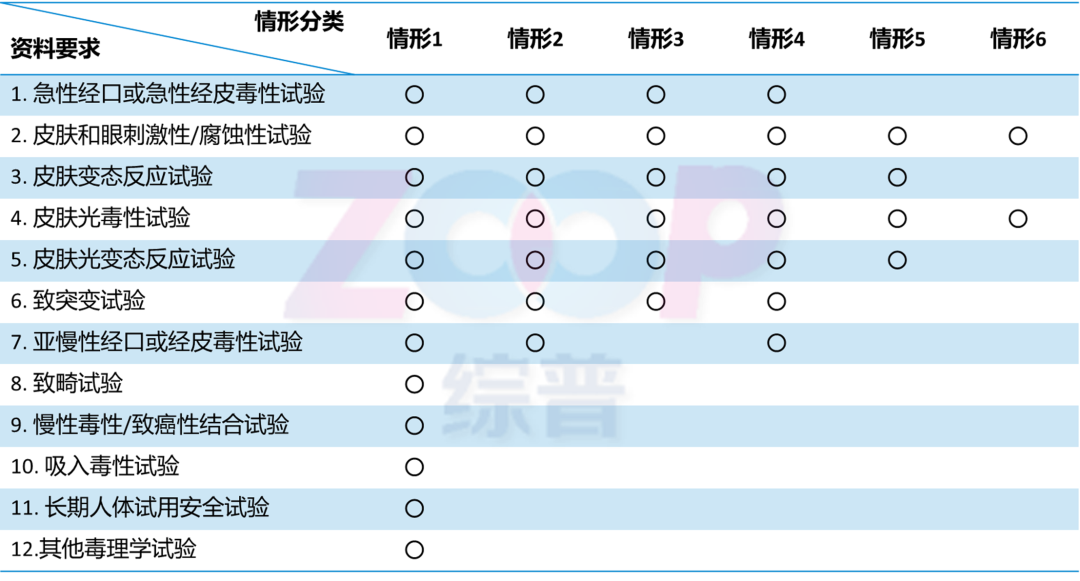
Regulations on the Management of Registration and Filing Materials for New Cosmetics Ingredients According to the characteristics of the raw materials function Usage History Consumption history and other related information,Classify new raw materials into different situations,And based on the concept of risk management, propose corresponding toxicology test project requirements.The function of raw materials and their consumption history are independent attributes,There is probably no correlation between the functions of the raw materials and whether they have a history of consumption.that is to say,The raw materials may have both spot removing and whitening functions,There is also a history of safe consumption.Although there is currently no new whitening ingredient with a history of safe consumption,But in the future, this possibility is not0.
Under what circumstances should new whitening ingredients with a history of safe consumption be declared
New raw materials with sufficient history of safe consumption can exempt some toxicology testing projects.haveSpot removal and whiteningNew materials for functionality belong to the category1,HavingSafe consumption historyThe new raw materials belong to the situation5,According to the situation1Or situation5The declared testing costs vary greatly.
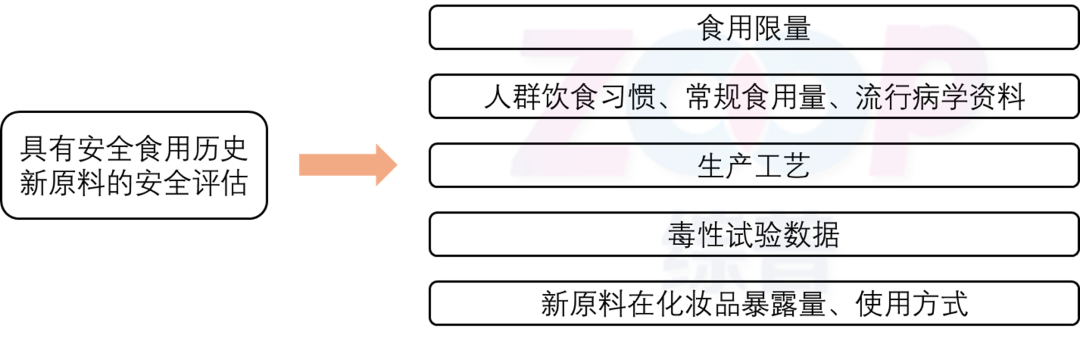
guide point out,For new ingredients with a history of safe consumption,Its safe consumption history can to some extent serve as available data information for toxicological endpoints related to systemic toxicity,Thus reducing the corresponding toxicology test items.
If the raw materials are according to the situation1declare,Its safe consumption history can also be reduced, including subchronic toxicity tests Teratogenic test chronic toxicity/Systematic toxicology tests including carcinogenicity binding tests,The registrant of new raw materials only needs to focus on local toxicity testing of the raw materials.After exempting the toxicity test of the system,Only remaining local toxicity tests and situations5The required toxicology tests are basically the same.In this situation,In addition to focusing on conventional local toxicity, guide Also pointed out that For new raw materials with spot removing and whitening functions,The history of safe consumption cannot exclude the safety risk of long-term use of it as a cosmetic ingredient on human skin,Should refer to Guidelines for Research and Determination of Safe Use History of New Cosmetics Ingredients trial Regarding the requirement to provide no less than100Long term famous consumers 1Year and above Relevant information on the safe use of cosmetics containing new ingredients in continuous use,Or supplement long-term human trial safety test research data.
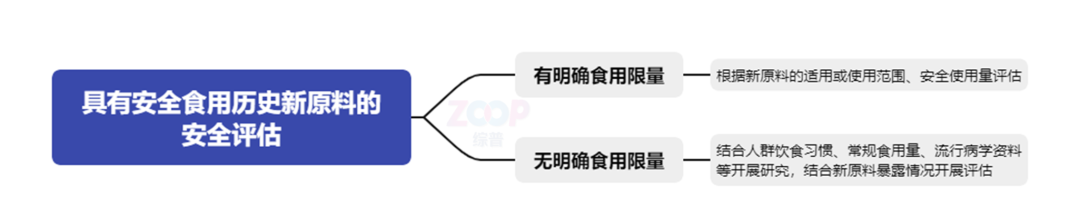
two Proof of safe consumption history or+New direction guide For those who are already within the territory of our countryNew raw materials for the sale of ordinary food ingredients on the marketProposed a new path for providing information For ordinary food ingredients that cannot obtain national food safety standards or public certification materials,Can explain the relevant situation,Provide relevant proof materials that have been listed and sold as ordinary food ingredients within the territory of China,And analyze and explain the situation of safe consumption, etc. guide Further clarification has been madeLocal specialty food ingredientsRequirements for declaring new raw materials for cosmetics The historical proof of safe consumption comes from local specialty food ingredients,Generally, there should be clear food safety standards,And there are no recognized health risks associated with consumption.Official certificates related to local specialty food ingredients should be provided,The current effective food safety standards formulated by relevant administrative departments at or above the provincial level Including local food safety standards . guide IncreasedRaw materials for health foodRequirements for declaring new raw materials The historical proof of safe consumption comes from the raw materials of health food,Proof materials issued by relevant national regulatory authorities should be provided,And combined with the requirements of health food management,For new raw materials intended for registration and filing Or its raw materials Thoroughly analyze the relevant situations that can be safely consumed. three Safe consumption×Safety Assessment New raw materials with a history of safe consumption,When conducting security assessments,Consider whether the raw materials have edible limits in food. guide point out There are clear consumption limits or other restrictions in food,It should be based on the applicability or scope of use of the new raw materials Information on safe usage, etc,Evaluate the safety of using new ingredients in cosmetics Without clear consumption limits,Can be combined with the dietary habits of the population Regular consumption amount Conduct necessary research on epidemiological data and other related information,And conduct a reasonable evaluation based on the exposure of new raw materials in cosmetics. at the same time,For extracts, guide clear The raw materials have a history of safe consumption,Proposed registration and filing of new raw materials in the form of their extracts, etc,During the exposure assessment process,Consideration should be given to converting the safe consumption levels of food raw materials. Evaluate based on the consumption limit or regular consumption amount in the history of safe consumption,Scope of use of new raw materials for registration and filing The safe usage amount cannot meet the demand for use in cosmetics,Can supplement repeated dose toxicity test data and other necessary data,And according to Technical Guidelines for Safety Assessment of Cosmetics Evaluate the program and requirements. food×New raw materials think If the scope of use of a certain food ingredient is clearly defined Excluding infant and toddler food ,When applying for a new cosmetic ingredient for this food ingredient,Is it necessary to clarify the applicability or scope of use of the new raw materials Excluding infant and toddler cosmetics It is known that children's cosmetics should not use new ingredients that are still under monitoring,Whether or not it is clearly applicable or within the scope of use of new raw materials Excluding infant and toddler cosmetics ,Children's cosmetics cannot add new ingredients that are currently under monitoring.however,If the new raw material has been included List of Used Cosmetics Ingredients ,Should the scope of application or use be clearly defined Excluding infant and toddler cosmetics New raw material services provided by Zongpu ◆ Guide new material registrants The registrant establishes a safety risk monitoring and evaluation system ◆ Based on the basic information of the new raw materials provided Research and Development Background Latest research progress,Assist in completing the report on the development of new raw materials ◆ Guide the applicant to develop a new raw material preparation process ◆ Guide the applicant in formulating quality control standards,Including stability test data Quality specification indicators and their inspection methods Possible safety risk substances and their control information ◆ Guide the applicant to conduct toxicological tests on new raw materials and prepare toxicological safety evaluation materials ◆ Collect relevant information on the efficacy basis of new raw materials,Including scientific literature regulatory information In vitro or animal experimental research data Human efficacy evaluation data, etc ◆ Guide the applicant to prepare technical requirements for new raw materials ◆ Guide the applicant to prepare an annual report on the safety monitoring of new cosmetic ingredients ◆ Registration of new raw materials Full process agency professional services for filing.