Product Compliance
filing/registration/consulting/training

The06/2011/TT-BYTnumber Regulations on Cosmetics Management
The93/2016/ND-CPRegulations on Production Conditions for Cosmetics
The07/2021/VBHN-BYTNumber Comprehensive Document Cosmetics Management Notice
containCircular 06/2011/TT-BYT and Circular 29/2020/TT-BYT
According to the ASEAN Cosmetics Directive B Part of the 2.1 strip,Cosmetics refer to products used for contact with external parts of the human body skin Hair system nail Lips and external genitalia Or teeth and oral mucosa,The main purpose is to clean Enhance fragrance Change appearance features form Adjust body odor Substances or preparations that protect the body or maintain its good condition .
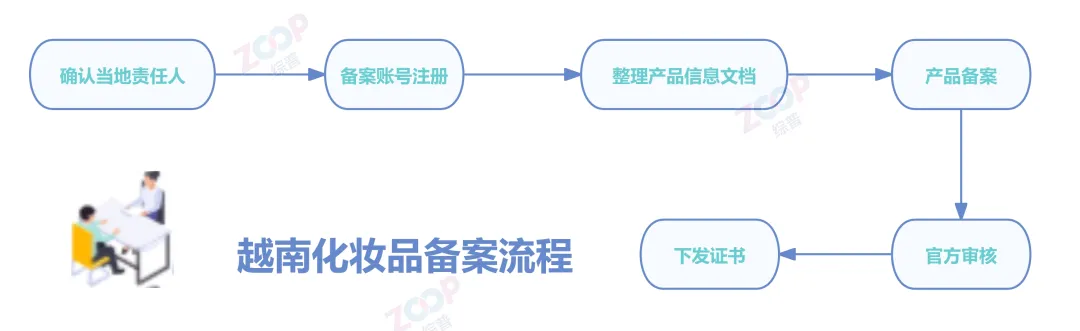
Preparation of Product Filing Documents
◆ product type
◆ Product Usage
◆ manufacturer,Packaging supplier,importer,Announcer,Exporting countries
◆ Product formula according to International Dictionary and Handbook of Cosmetic Ingredients List the ingredients INCI Name and content, etc
◆ certificate of authorization (Letter of Authorization, LoA)
Used to clarify the authorization relationship between product manufacturers or exporters and agents or distributors in Vietnam,Including basic information of both parties Scope of Authorization Key terms such as authorization period.This document must be written in Vietnamese or English,And authenticated by the Vietnamese Embassy of the issuing country.
◆ Free Sale Certificate(Certificate of free sale, CFS)
To the China Council for the Promotion of International Trade CCPIT Or relevant industry associations apply for a certificate of free sales.Product description must be submitted when applying Certificate of quality Production license Export license and other documents,After passing the review, a certificate will be issued.The language must be Vietnamese or English.
◆ Import company's business license Must have the qualification to operate cosmetics
◆ Additional information and documents
![]() It cannot be a claim for treating or preventing diseases
It cannot be a claim for treating or preventing diseases
![]() The effect of the product must be non permanent
The effect of the product must be non permanent
![]() After obtaining the registration and filing number,Enterprises need to establish and save product information files,Including administrative document summaries product quality Material quality Content related to safety and efficacy.
After obtaining the registration and filing number,Enterprises need to establish and save product information files,Including administrative document summaries product quality Material quality Content related to safety and efficacy.
Zoop’s services
◆ Account Registration
◆ Vietnam Cosmetic Notification
◆ Formula and Label Review
◆ Product Safety Assessment Reports
◆ Review and Collation of Product Information Files (PIF)
◆ Local Agent