Product Compliance
filing/registration/consulting/training

Regulatory background
2023 year 11 month 1 day,The US Food and Drug Administration (FDA) release Guidelines for Registration of Cosmetics Facilities and Listing of Products Official version,yes 2022The Modernization Act of Cosmetics Regulations (MoCRA) More detailed explanations have been provided on the requirements for facility registration and product listing in China.Cosmetics exported to the United States require facility registration and product listing.
*Facility registration: Facility registration,It can be simply understood as factory registration,The following will use factory registration instead of facility registration,This is to clarify that.
Before officially initiating the process of product compliance,It is necessary to first have a deeper understanding of which departments regulate cosmetics in the United States.Cosmetics, as a product closely related to people's daily lives,Its regulation is crucial.Clarifying regulatory authorities helps companies better ensure that their products comply with various regulatory requirements,Protecting the rights and safety of consumers.therefore,Understanding the regulatory authorities for cosmetics in the United States in advance is an important prerequisite for carrying out product compliance work.


Federal Food Drug and Cosmetic Act FD&C bill Define cosmetics as Expected to apply dump spray Spray or other methods for human body,Can clean up beautify Products that enhance attractiveness or change appearance purpose ,Including moisturizer perfume lipstick nail polish Eye and facial cosmetics Cleaning shampoo Perm agent hair dye Deodorizers and any substances intended as cosmetic ingredients [FD&C bill,The 201 i section].The products included in this definition include skin moisturizers perfume Lipstick nail polish Cosmetics Cleansing shampoo Perm and curling agent Hair dye and deodorant,And any substance intended for use as a cosmetic ingredient.

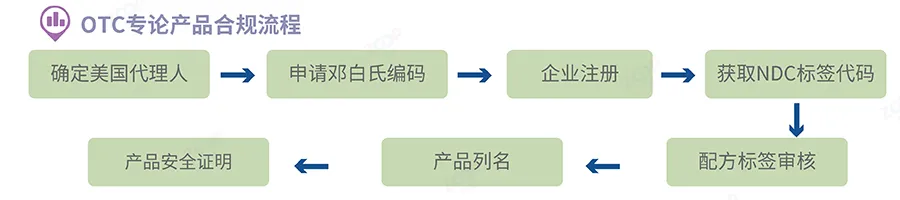
Preparation of factory registration documents
factory Ordinary cosmetics Enterprises involved in the production and processing of cosmetics for sale in the United States Including overseas enterprises
factory OTC All engaged in production Repackaging Re label or recycle drugs,Enterprises used for commercial sales mustFDAregister.
◆ The owner of the factory and/Or the name of the operator
◆ The name of the factory Actual address Email address and phone number
◆ For any factory located outside of the United States,Contact information of the US agent of the factory Name and phone number ,Electronic contact information E-mail,If there is, then
◆ enterpriseFEIRegistration Number,Obtain the specifiedFEIEncoding is the first step in submitting factory registration
◆ Previous factory registration number(If there is),Exemption is possibleFEIRegistration Number
◆ The brand name of the cosmetics produced or processed by this factory
◆ Enterprise Dun&Bradstreet Code OTC
◆ Product category and responsible person for each type of cosmetics manufactured or processed in the factory
◆ Type of submission initial modify Renewal every two years or simplified renewal
* In addition, there are some non mandatory information submissions,For example, parent company information, etc.
person liable Ordinary cosmetics : Cosmetics manufacturers that appear on cosmetic labels Packaging supplier or distributor
person liable OTC manufacturer Repackaging Re label or self branded distributor Private label distributor ,PLD .
◆ factoryFEIcode
◆ Enterprise Dun&Bradstreet Code OTC
◆ Name and contact number of the responsible person Consistent with what is shown on the label
◆ Product Name Consistent with what is shown on the label
◆ Product Category Refer to guide The attachments provided in the documentA
◆ List of ingredients in cosmetics,Including essence spice Coloring agent,Each ingredient is prepared according to21 CFR 701.3Identify the usual names of the required names or ingredients in the section
Product Listing Number(If there had been before)
◆ Type of submission initial Content Update year Simplify Renewal .
* In addition, there are some non mandatory information submissions,Like a label Product website link Whether the product is only used for professional purposes, etc.
ZOOP'S SERVICES
◆ Facility registration of cosmetics and FDA OTC monograph products
◆ Product formula and label review
◆ General cosmetics and OTC specialised products listing
◆ Annual update service of facility registration and product listing
◆ Testing and safety assessment services required for product safety certification
◆ Undertake local agency in the United States (as needed)
◆ Cosmetics Classification Definition Service
◆ U.S. Cosmetics Regulatory Conformity Training
◆ Other customised services for customers