Product Compliance
filing/registration/consulting/training

What are freckle removal and whitening products
The color of human skin is mainly determined by the types of melanin in the skin Quantity and distribution determine.according to Classification rules and catalog for cosmetics ,Spot removing and whitening cosmetics mainly refer to those that help reduce or slow down skin pigmentation Cosmetics that achieve skin whitening and brightening effects,Products that improve acne scars caused by pigment deposition should also be declared as spot removing and whitening products.
Only achieving skin whitening and brightening effects through physical covering,Declarable Spot removal and whitening products Only has a physical covering effect .
Only by increasing the degree of hydration clean Exfoliating and other methods,Improving skin brightness or accelerating keratin shedding and renewal,There are differences in the main mechanism of action between freckle removing and whitening cosmetics,Not classified as spot removing and whitening cosmetics.
Only sunscreen is used in the formula Products without the use of spot removing and whitening agents,Declarable Sunscreen category ,Cannot declare simultaneously Spot removal and whitening products ,This type of product can claim to help alleviate skin darkening caused by sun exposure Pigmentation,Do not directly claim the effect of removing spots and whitening.
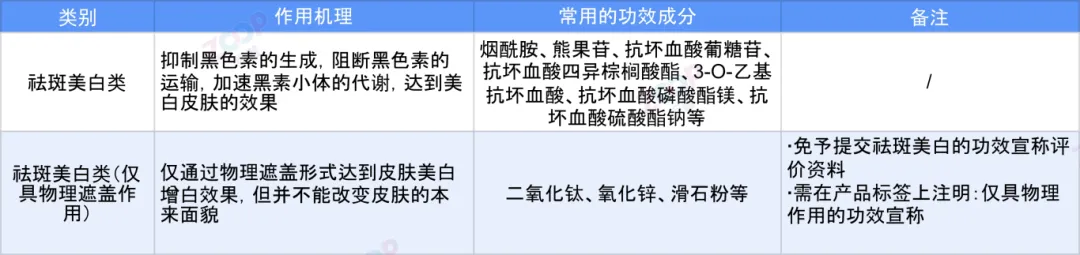
According to the mechanism of action,Spot removing and whitening cosmetics are divided into physical whitening products Spot removal and whitening products Only has a physical covering effect Chemical whitening Spot removal and whitening products Two types.
Pre market requirements for spot removing and whitening cosmetics
Due to the relatively high risk level of spot removal and whitening products, Regulations on the Supervision and Administration of Cosmetics It is clear that it belongs to the category of special cosmetics and requires registration with the drug regulatory department of the State Council before production can be carried out Import.
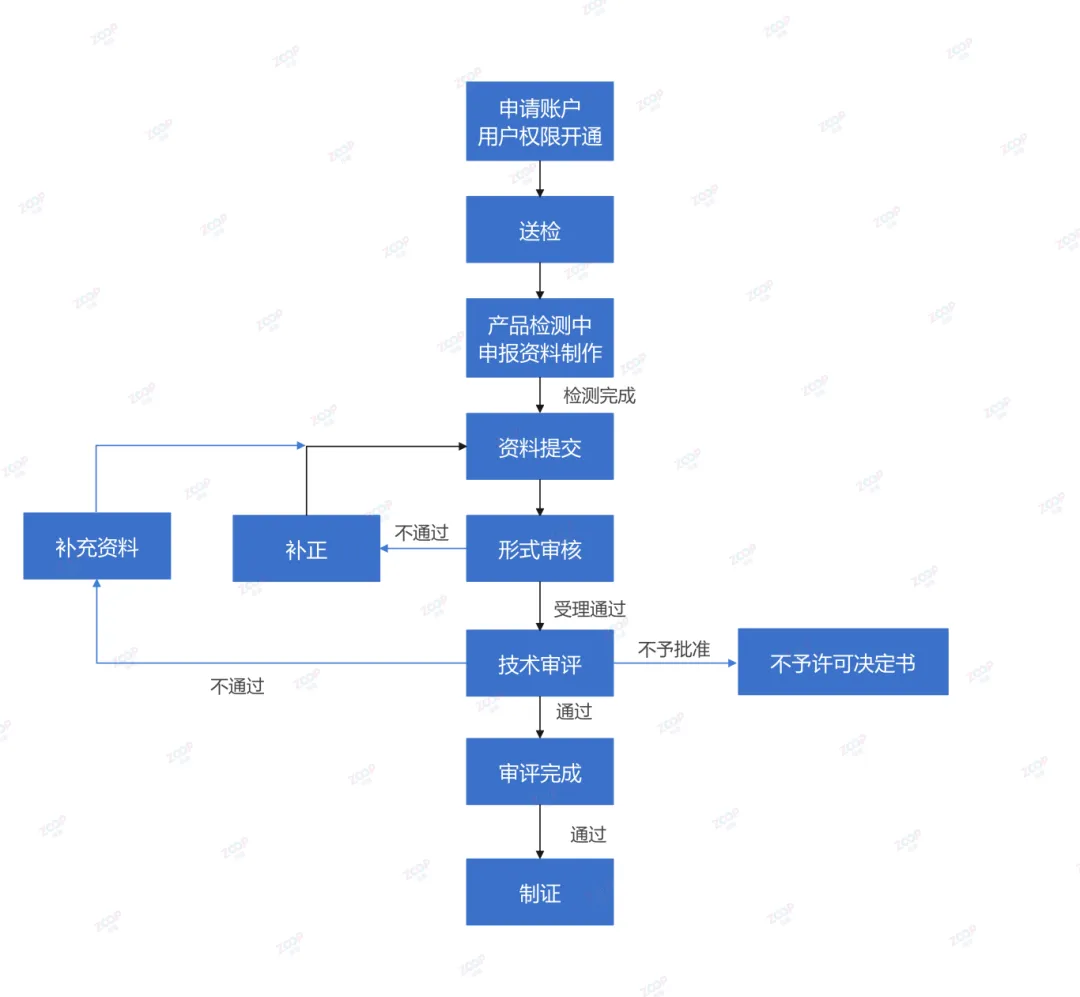
Registration Process and Cycle for Spot Removing and Whitening Cosmetics
cycle
Physical whitening declaration cycle about7-11month
Chemical whitening declaration cycle about10-13month
Required Information for Registration of Spot Removing and Whitening Cosmetics
◆Cosmetics Registration and Filing Information Form
◆Naming criteria for Chinese product names
◆Product formula Contains all raw material safety information
◆Standards for product execution
◆Product label sample
◆Product Inspection Report
◆Product safety assessment data
◆Summary of Other Efficacy Claims of the Product if there be
◆Other information that may help with registration.
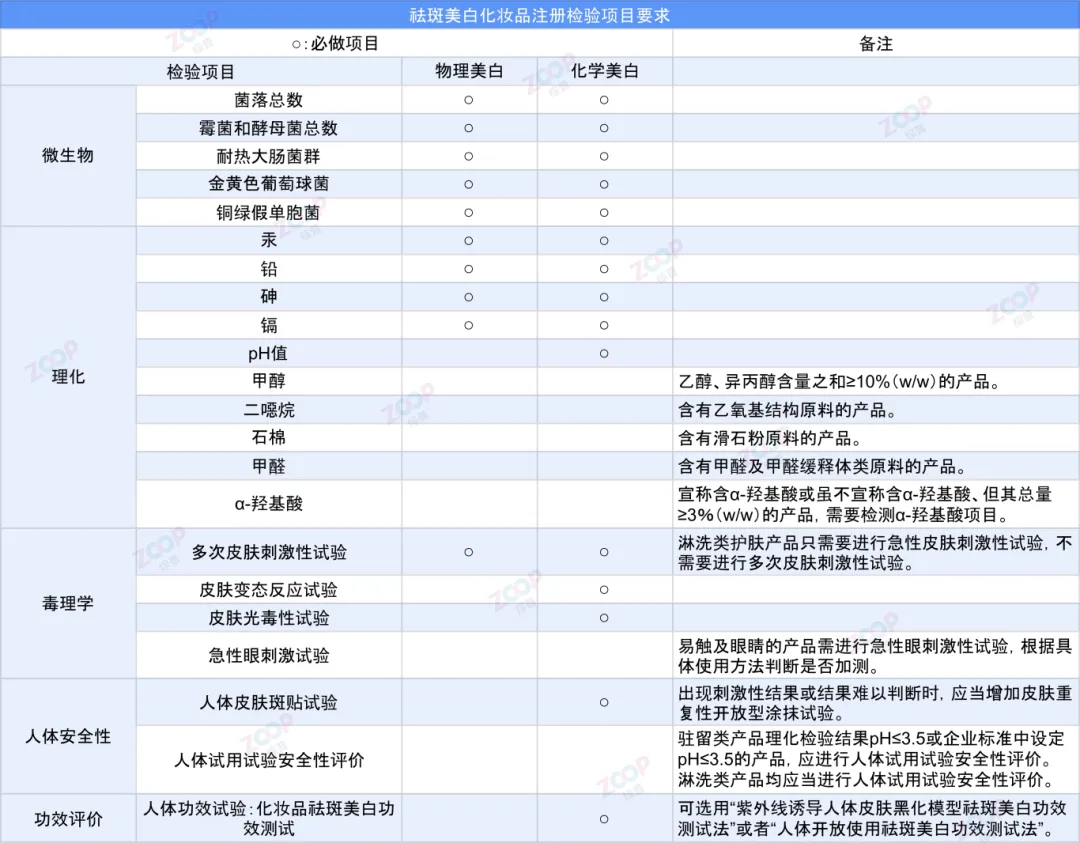
Requirements for Registration and Inspection Items of Spot Removing and Whitening Cosmetics
Key points of declaration Difficulty analysis
In the product formula of spot removing and whitening cosmetics,Clear depigmentation and whitening agents should be reported.If it's not a single component,The specific whitening and freckle removing ingredients should be clearly stated in the purpose column of the formula table.
1.When applying for spot removing and whitening cosmetics that only have a physical covering effect,Chemical whitening ingredients should not be added to the product formula.If the product formula contains chemical whitening ingredients,The product may actually have a chemical whitening effect,This will be in line with the claimed Only physical covering Conflicting effects.
2.The use of spot removing and whitening agents should be scientific and reasonable,Except for newly approved cosmetic ingredients that can be used as spot removing and whitening agents,A certain usage basis should be provided,Explain the rationality of its use as a spot removing and whitening agent.
So how should the basis for the ingredients of freckle removal and whitening effects be provided
Applicants for cosmetics registration can choose based on their actual research and development situation,Choose at least one of the following two types of criteria to provide
The first type is regulatory materials published through regulations or approved by regulatory authorities for use as raw materials for freckle removal and whitening effects.among,For non single component raw materials such as plant extracts,There should be specific functional ingredients that can effectively remove spots and whiten the skin.
The second type is the scientific basis and efficacy evaluation report of the mechanism of action of raw materials.
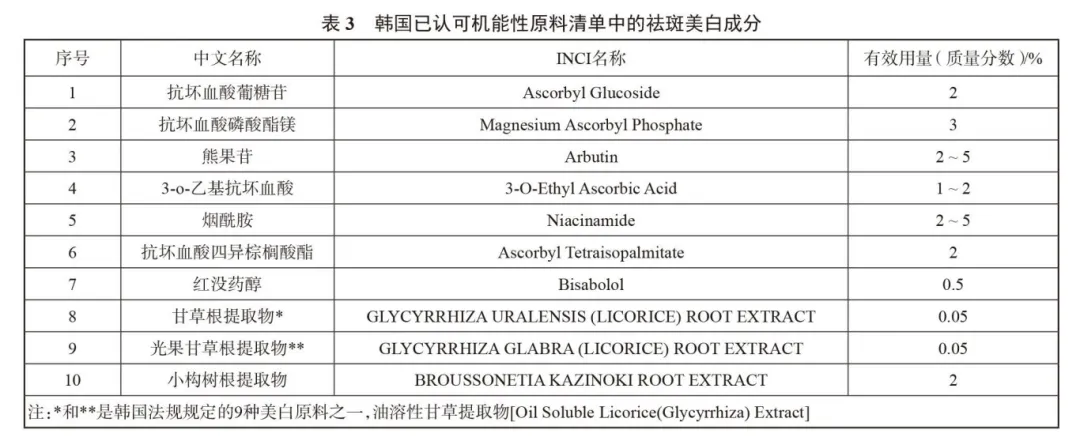
At present, there is no official list of effective ingredients for freckle removal and whitening in China,For the selection of chemical whitening ingredients,Comprehensive recommendation can refer to the Korean whitening efficacy ingredient list.
Warm Tips
The validity period of the registration certificate for spot removing and whitening cosmetics is5year,The registrant shall obtain the product registration certificate before its expiration date90From one working day to30Submit an application for renewal of registration within one working day.
Zoop not only provides comprehensive registration and declaration services,Also includes product labels&Formula compliance audit Compliance analysis of raw materials Safety evaluation data analysis Product changes and extended services before expiration.
Zoop has 13 years of product compliance experience,Experienced technical teachers in the team,We can provide one-stop detailed declaration services for enterprises,Have relevant needs or questions,Welcome to contact us in a timely manner.
The services provided by Zoop
Full process application agency service for whitening and other special certificates
Formula and label compliance review service
Raw material and safety evaluation data analysis services
Technical support services for rectification of application opinions.