Product Compliance
filing/registration/consulting/training

Since the implementation of the policy on new raw materials for cosmetics,Raw material suppliers and industry practitioners are generally concerned about two core issues regarding new raw materials "Can this raw material be declared as new " as well as "What toxicology tests are required for new raw materials " Regarding these issues,We provide comprehensive answers and guidance in this article.
What are new cosmetic ingredients
Definition of new cosmetic ingredients stayFor the first time in China, it has been used in cosmeticsNatural or artificial ingredients used as new cosmetic ingredients.
Adjust the purpose of using cosmetic ingredients that have already been used Safe usage, etc,Should be registered according to new raw materials Filing requirements: Apply for registration Record filing.
Classification management of new cosmetic ingredients
Record management Other new cosmetic ingredients are subject to filing management.
Determination of new cosmetic ingredients
Those who meet one of the following conditions,Belonging to new cosmetic ingredients
1.Natural or artificial raw materials used for cosmetics for the first time in China, And its expected usage method Application site The purpose of use falls within the scope of the definition of cosmetics.
2.Adjust the use purpose or safe usage amount of cosmetic ingredients that have been used.
3.Although registered or filed,But it has not been included yet List of Used Cosmetics Ingredients The raw materials.
Raw materials that meet one of the following conditions,Not belonging toNew raw materials for cosmetics
1.Included in List of Used Cosmetics Ingredients 2021Year Edition The raw materials.Cosmetic registrant When selecting raw materials from this catalog, the registrant,Should comply with relevant national laws and regulations Mandatory national standards Requirements related to technical specifications,And bear the responsibility for product quality and safety.If you need to exceed Highest historical usage When in use,Should be in accordance with Technical Guidelines for Safety Assessment of Cosmetics The program and requirements demonstrate its safety.
2.Specific raw materials included in the used category of raw materials.The category of raw materials has been included in the directory collagen ,Namely collagen protein,Refers to a general term for a certain category of raw materials,This category of raw materials includes different process sources such as animal tissue extraction Gene recombinant collagen,It also includes different subtypes such asIType collagen IIICollagen, etc.in addition, List of Used Cosmetics Ingredients 2021edition Included in Extract from a certain plant raw material,for example ginseng extract Indicating that both the whole ginseng plant and its extract are used raw materials,If declared separately Ginseng juice Or if a specific part of ginseng is a new raw material,Will not be accepted.
3. Technical specifications for cosmetic safety Raw materials that have been designated as prohibited components.Like human cells Organizational or human source products Antihistamines Hormonal substances, etc.
4.Raw materials with actual functions beyond the defined scope of cosmetics.If possessing Activate cells Regenerated cells Reduce pigment deposition at the wound site Promoting healing effect Promote the discharge of heavy metals Waiting for raw materials with medical effects.
Toxicological testing requirements for different situations
The current regulations classify new cosmetic ingredients into the following six situations
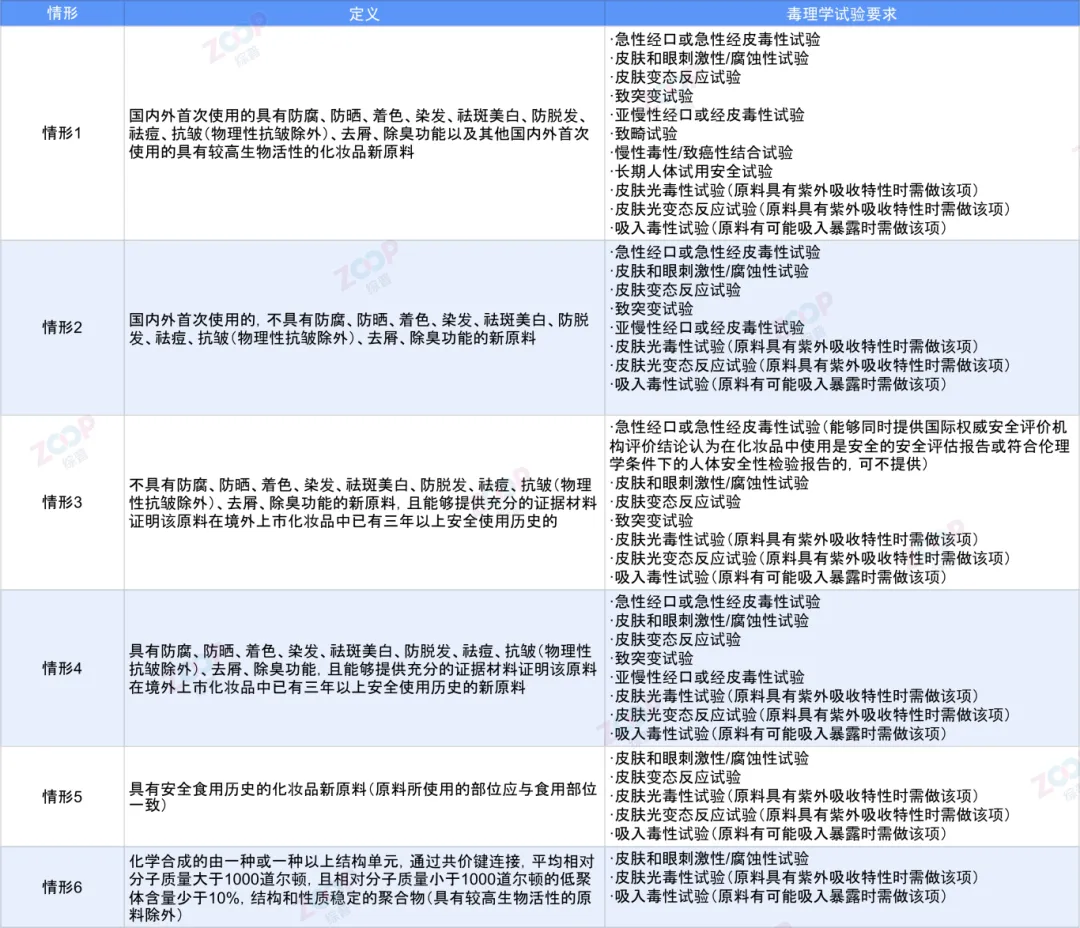
Differences in the scope of the situation
Whether the raw materials have the following functions anticorrosive Sunscreen to color dye one 's hair Spot removal and whitening Prevent hair loss acne treatment Anti wrinkle Excluding physical wrinkle resistance Dandruff removal Deodorization.
2.situation1And the situation4The difference situation2And the situation3The difference
Whether the raw materials have been safely used in cosmetics listed overseas for more than three years.
Toxicological differences in different situations
1. Risk level and toxicology testing requirements
situation1Having the highest risk level,Therefore, the most rigorous toxicology tests are required.This has resulted in significantly higher testing costs compared to other situations,Probably the situation2More than ten times the cost.
2.situation3In relation to the situation2The comparison
situation3Compared to other situations in toxicology testing2Missing an important experimental data item Subchronic oral or dermal toxicity test.
3.situation4In relation to the situation2The similarity
situation4In relation to the situation2Although there are differences in the scope of application,But the toxicology test data required to be submitted is consistent.
4.situation5In relation to the situation2The difference
situation5Compared to the situation2Three key toxicological test data are missing,Including acute oral or acute dermal toxicity tests Mutation test and subchronic oral or dermal toxicity test.
5.The situation where the minimum toxicity test is required
among,situation6If there is no UV absorption characteristic and inhalation exposure risk,Just need to do skin and eye irritation tests,And this toxicology test not only has a short cycle,And the cost is relatively low.In relation to the situation5comparison,situation6Two toxicology test data are missing Skin allergy test and skin photoallergy test.
Based on the above differences,We can see the differences in toxicology testing requirements and costs between different situations.
Toxicological testingQ&A
Q Acute oral or dermal toxicity test,It's an acute oral toxicity test Do acute transdermal toxicity tests need to be conducted A Choose one from two. Q: Do we need to do both skin irritation and eye irritation tests A Everything needs to be done. Q. What tests are included in mutagenicity testing A At least one genetic mutation test and one chromosomal aberration test should be included. Q: Do inhalation toxicity tests need to be conducted A Inhalation exposure risk assessment should consider both the physical and chemical properties of the raw materials themselves,When using raw materials in cosmetics, it is necessary to combine them with the product formulation Factors such as usage methods. Q: Subchronic oral or dermal toxicity test,You can choose28Is there a dermal or oral toxicity test A may not. |
ZOOP'S SERVICES